Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 28 August 2024
A qualitative exploration of the patient journey in axial spondyloarthritis towards a people-centered understanding
- Kristina Berr 1 ,
- Stefanie Ziehfreund 1 ,
- Martin Welcker 2 ,
- Tilo Biedermann 1 &
- Alexander Zink 1 , 3
Scientific Reports volume 14 , Article number: 19977 ( 2024 ) Cite this article
Metrics details
- Health services
- Spondyloarthritis
This exploratory qualitative study aims to gain a people-centered understanding of the patient journey in axial spondyloarthritis (axSpA). Semi-structured interviews were conducted with 15 individuals diagnosed with axSpA, aged 18 years and older, who were purposively recruited from a rheumatologic practice in southern Germany. The interviews were carried out as web-based video calls between September and October 2021, audio-recorded, transcribed verbatim, and analyzed according to Kuckartz’s qualitative content analysis. Patient journey narratives encompassed both healthcare journeys and personal journeys. Healthcare journeys were characterized as fragmented and difficult to navigate, with diagnosis often marking a turning point toward more coordinated care. Post-diagnosis, new challenges emerged (e.g., time management for treatment). Personal journeys comprised perceptions of axSpA in social contexts (e.g., stigmatization) and the continuous interplay of comorbidities and biographical events with healthcare related to axSpA. This study proposes a people-centered perspective on the patient journey in axSpA, emphasizing the interplay of biographies, comorbidities, and social context with healthcare events. Recognizing these personal factors in clinical practice is encouraged to address complex health needs and tailor treatment to each individual. Further efforts should promote collaboration between medical disciplines and integrate healthcare and social support at all stages of the axSpA patient journey.
Similar content being viewed by others

Qualitative study identifies life shifts and stress coping strategies in people with multiple sclerosis
A data set of symptoms and needs of individuals affected by COVID-19

The process of social death in patients with hip fracture
Introduction.
Axial spondyloarthritis (axSpA) is a chronic rheumatic disease marked by inflammatory back pain as a leading symptom, but can also be accompanied by joint pain, stiffness, fatigue, and reduced physical function 1 . Besides the spinal and sacroiliac joints, axSpA presents as a multisystemic inflammatory disease with manifestations extending to peripheral joints, entheses, skin, bowel, and eyes 1 . Affected individuals not only deal with these physical symptoms but also confront a range of impacts on their daily life, occupational responsibilities, social interactions, mental well-being, and overall quality of life (QoL) 2 , 3 .
Effective multimodal treatment strategies, as established and outlined in guidelines 4 , are crucial for preventing irreversible structural damage, preserving spinal mobility, and enhancing QoL 5 . However, there is often a substantial delay between symptom onset and axSpA diagnosis with 5 to 14 years in average 6 due to underrecognition of symptoms among non-rheumatology health providers and the lack of well-established diagnostic tools 7 , 8 . Furthermore, the management of individuals affected by axSpA requires a coordinated approach between rheumatologists and other specialists such as dermatologists, gastroenterologists, cardiologists, radiologists, physiotherapists, etc. 9 . This collaborative approach involves both non-pharmacological and pharmacological treatment interventions tailored to the current signs and symptoms of the disease and patient characteristics 9 . Given the current fragmented healthcare system 10 , 11 , timely, integrated, and people-centered care where healthcare professionals work together to achieve a person’s life goals and individual’s responsibility, is encouraged. This approach aligns with the World Health Organization’s definition of quality health services 12 .
Patient journey mapping activities provide an opportunity to improve people-centered health solutions 13 . Patient journeys assess care processes over time from the patient’s perspective rather than pin-pointing specific care events within individual healthcare facilities 14 . While there is a growing body of healthcare research using the patient journey approach, there is no uniform framework for applying it 14 , 15 . Typically, patient journeys are presented as chronological stages or touchpoints with the healthcare system 16 ; however, some studies used different visualizations like circular mind maps 17 or Aboriginal artworks 18 . Others have expanded the focus of patient journeys beyond healthcare touchpoints, for example exploring the broader “life journeys” of teenagers with chronic diseases 19 or incorporating “disruptive life events” in patients’ journeys with colorectal cancer according to people-centered care 17 . Previous studies on axSpA primarily focused on the journey to diagnosis, particularly on the reasons and impact of delay 20 , 21 , 22 . Thus, this study aims to gain a more comprehensive understanding of the intricate patient journeys experienced by individuals with axSpA, exploring the broader personal context.
Study design
For this exploratory qualitative interview study, semi-structured interviews were conducted with 15 patients diagnosed with axSpA. The participants were recruited at a rheumatology practice in Bavaria, southern Germany, and interviewed between September and October 2021. Inclusion criteria were 18 years of age and older and confirmed diagnosis with axSpA. Exclusion criteria were inadequate mental or legal capacity and insufficient knowledge of German. The study adhered to the ethical standards of the Declaration of Helsinki and was reviewed and approved by the ethics committee of the Medical Faculty at the Technical University of Munich (reference 210/21 S-EB). Reporting adhered to the consolidated criteria for reporting qualitative research (COREQ) 23 . All participants were informed about the voluntariness of the study and gave written informed consent prior to their interviews. Although patients and the public were not explicitly involved in the design and conduct of the study, efforts were made to incorporate patient perspectives throughout the study. This included reviewing patient forums and podcasts, allowing flexibility in interview duration, and pilot testing the interview guide with a patient.
Recruitment and participants
The investigation was planned to continue until data saturation was reached 24 . Drawing on previous experience with qualitative studies, a larger number of patients were initially contacted to ensure sufficient participation. In May and June 2021, invitations were mailed to 31 patients with an axSpA diagnosis registered at the rheumatology practice who had previously consented to receiving study invitations. These patients were selected through purposive sampling to achieve demographic heterogeneity in terms of age, gender, and living distance to the rheumatology practice. After the mailing, the interviewer (KB, female, medical student trained in qualitative interviewing) conducted personal phone calls with potential participants to gauge their interest in participating, discuss study details, and address questions.
Data collection
A semi-structured interview guide with open-ended questions was designed according to Helfferich’s manual for conducting qualitative interviews 25 by KB and reviewed by SZ and AZ (Supplementary Table S1 ). In an initial openly posed question, participants were invited to narrate the story of their patient journey. This approach allowed participants to freely express their experiences and perspectives, thereby providing rich, qualitative data that revealed insights into their personal and emotional encounters. To follow up, the interview guide was structured along chronological steps outlined in previous empirical and conceptual work on patient journeys 2 , 16 , 21 , 22 , 26 , 27 . Specifically, the provided questions and prompts focused on the onset of the disease, the journey to diagnosis, and treatment and ongoing care.
The interview guide was pretested twice (first with a healthy person, then with patient P1, both providing feedback). Since only minor adjustments were made and the main guiding questions were maintained, the pilot interview with P1 was included in the analysis. The participants did not know the interviewer prior to the study and were informed of her name, qualifications, and research objective. Interviews were conducted as web-based video calls using telemedical software which was considered a valid alternative to in-person interviews during the COVID-19 pandemic 28 , 29 . Interviews were carried out in a quiet environment with no other persons present, except for a brief interruption by a family member of P8 and a ringing phone during the interview with P3. The interviews were audio-recorded, transcribed verbatim, and anonymized and the interviewer’s impressions and reflections were documented in postscripts. No repeat interviews were conducted. With interviews 13 to 15, the narratives seemed to become increasingly congruent, and no new topics emerged. Thus, data saturation was considered to be reached according to the interviewer’s subjective judgment 24 .
Data analysis
The interview transcripts were analyzed based on Kuckartz’s qualitative content analysis in an iterative process comprising five phases: 1) initiating text work 2) developing the coding frame 3) coding the data 4) analyzing the coded data, and 5) presenting the results 30 . Going back and forth between literature 2 , 21 , 26 , 27 , 31 and interview data, the coding categories were continuously refined and rearranged by KB and SZ. The final coding frame was developed through consensus among the research team after nearly half of the data had been coded (Table 1 ). Using the final coding frame, two interviews were coded independently by two researchers (KB, SZ). The intercoder reliability was calculated using Cohen’s kappa (0.92). MAXQDA 2022 (VERBI Software) was used for transcription and analysis.
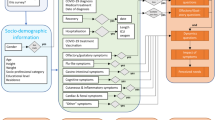
The study included 15 participants, comprising 9 women and 6 men (Table 2 ). Reasons for non-inclusion were disinterest in participating (n = 8), difficulty establishing or maintaining contact (n = 6), and mismatch in inclusion or exclusion criteria (n = 2). Participants ranged in age from 24 to 69 years, with a median age of 40 years. Interview durations varied from 43 to 157 min with a median duration of 60 min. Initially, the analysis focused on the chronological axSpA patient journey through the healthcare system, with the overarching category ‘ C1 healthcare journey ’. However, the highly explorative interviews elucidated that linear and single-disease centered perspectives of the axSpA patient journey did not meet the participants’ lived experiences. Thus, the second overarching category ‘ C2 personal journey ’ emerged inductively during the coding process. Participants narrated their patient journeys in more complex ways, intertwining experiences with the healthcare system and their personal biographies, comprising life events, social contexts and comorbidities, not all of them directly related to axSpA. Figure 1 illustrates the evolution of the applied patient journey approach from a linear timeline (as seen in previous work 16 , 26 , 27 ) to intertwined strands. The analysis was narrowed down to four subcategories of interest: For ‘healthcare journey’, the analyzed subcategories were ‘navigation hurdles’ and ‘diagnosis as turning point’. For ‘personal journey’, the subcategories were ‘self- and external perceptions of axSpA’ and ‘biographical factors and comorbidities as context factors’.

Explorative development of the patient journey approach applied in this study. ( a ) Initial approach: Focus on the healthcare journey as a linear timeline. Based on previous literature on patient journey mapping 16 , 27 and the diagnostic journey in axial spondyloarthritis 2 , 21 , 22 , 26 , the initial approach to interview conduction and data coding was a linear timeline with chronological stages through the healthcare system. ( b ) Evolved approach: Patient journey as intertwined strands. Throughout the highly iterative, explorative research process, an interplay of healthcare experiences and personal context factors emerged. An evolved patient journey approach was adopted that intertwines healthcare and personal journeys, encompassing comorbidities, biographies, and perceptions of axSpA in social contexts as integral context factors. The figure illustrates the interplay of healthcare journey and personal journey with exemplary life events, comorbidities, and social context.
C1 healthcare journey
C1.1 navigation hurdles.
Navigating the healthcare system was predominantly described as unintuitive and arduous. Participants described their healthcare journey as an ongoing learning process: “I didn’t keep a lot of documents that would have been important because I didn’t know they were important. I completely lacked this information […] I had to learn all that first.” (P13). This was especially reported by previously healthy participants who had not been in close contact with healthcare services before. Participants with previous experiences due to comorbidities or work in the healthcare system emphasized navigation hurdles due to axSpA less prominently.
Different logistical hurdles on the patient journey to timely and satisfactory healthcare were discussed, such as a lack of specialists leading to long travel distances, extensive waiting and referral times as well as the need to take on unfamiliar administrative tasks (e.g., dealing with medical records).
In addition to these logistical hurdles, participants emphasized the exhaustion of having to navigate a fragmented healthcare system, pointing out that “the patient can’t always know where he actually needs to go, because it’s not always logical” (P6). Many participants wished for consistent and professional coordination of their healthcare journey but instead felt overwhelmed by having to advocate for themselves. For example, P14 found herself in the role of a “spokesperson” or “translator” between doctors who failed to network with each other.
Aside from having to coordinate within a fragmented healthcare system, participants perceived short-term, symptomatic interventions as hurdles on their diagnostic journey: “They just inject you, then they don’t want to see you again.” (P1). The lack of consistent care in the diagnostic process was criticized as a reason for diagnosis delays and as a source of emotional distress: “Nobody really commits to anything concrete […] It’s simply stressful when you’re always left with a ‘maybe’.” (P6).
C1.2 diagnosis as turning point
In most interviews, the diagnosis was narrated as a turning point in the adverse patterns and beliefs that had permeated the pre-diagnostic journey. One participant described this as a moment of forward energy: “[…] now the diagnosis is made, that’s our basis for work, now let’s do it, let’s go!” (P6).
The diagnosis changed the narrative of the healthcare journey in different regards. Firstly, participants described a shift from self-directed to expert-led care, alleviating feelings of overwhelm and isolation. Participants reported feeling “helpless” (P2) and “left alone” (P15) prior to the diagnosis and noted a positive change upon having a designated contact person in the diagnosing rheumatologist.
Participants further expressed a sense of reduced uncertainty due to diagnosis, now having access to targeted treatment options instead of trial-and-error approaches: “As soon as you know something, you can do something about it. If you don’t know anything, you just try this and that and you just feel like a guinea pig.” (P9).
In addition, the diagnosis validated previously inexplicable symptoms and was described as a relief from prior discreditation and stigmatization. Symptoms that had frequently been misattributed to factors like obesity or inactivity and mislabeled as “psychosomatic” (P2) or “hypochondriac” (P5) were now appropriately addressed.
While the diagnosis was reported as reducing burdens of uncertainty and allowing for better treatments, it also was discussed with a range of concerns. For instance, a younger participant noted that being diagnosed with a chronic condition shortly after entering her first job felt like she “lost the perspective for the future ” (P14). Another participant highlighted issues with how the diagnosis was communicated, stating, “I never really got a diagnosis, it was just written on the paper at some point. ‘Axial spondyloarthritis’.” (P13). Moreover, the diagnosis introduced time-consuming multimodal treatments, including physiotherapy, doctor visits, and lifestyle changes, which was discussed in conflict with work, family, children, and household chores.
C2 personal journey
C2.1 self- and external perceptions of axspa.
Most participants reported about the high burdens of disease perceived and stressed the broad impact of untreated axSpA on everyday life, employment and financial status, social relationships, and caregiving roles: “It was quite sobering to realize that I couldn’t do anything and couldn’t lead my normal life. I used to go to sports, then take care of my university stuff, and maybe go to work afterward, but all of that was simply not possible.” (P4). The unpredictability of symptom flare-ups and daily energy levels led some participants to reduce leisure activities and adopt a more restrictive lifestyle.
AxSpA was often described as an externally invisible disease. As a consequence, participants encountered misunderstanding and disbelief . Especially young, healthy-looking participants reported misunderstanding from peers: “My peers could never quite understand that, nor could they understand this change: One day I’m jumping around and can do everything, and the next day I can’t do anything.” (P14). Younger participants also expressed confrontation with the misconception that rheumatic diseases primarily affect older people. Another stigmatizing association mentioned in the interviews was that of back pain with an inactive lifestyle or being a “couch potato” (P10). To evade this stigma as an overweight person with axSpA, one participant described adopting postures in front of others to conceal the back pain.
These reports illustrate a tension between self-perception and external perceptions of axSpA, with participants expressing a desire for greater recognition of their invisible condition while simultaneously feeling the need to avoid negative attention or stigmatization.
C2.2 biographical factors and comorbidities as context factors
The interviews revealed a dynamic interplay between participants’ healthcare journeys and their personal journeys. Throughout their narratives, healthcare directly related to the diagnosis of axSpA was oftentimes confounded with broader personal journeys, and in some cases, conflicting interests emerged.
On the one hand, axSpA was frequently described as a disruption to participants’ daily lives, family or career plans, and future perspectives. On the other hand, participants described how biographical factors and life events could amplify the burden of axSpA: “At the same time that fall, my grandmother died, who I got on very well with, and my boyfriend at the time broke up with me. In other words, my psyche was on a bit of a rollercoaster. So, I was in hospital for a short time and was also given medication, and I then received therapy.” (P14). This illustrates how biographies were narrated in mutual interplay with patient journeys.
Similarly, comorbidities emerged as contextual factors shaping the axSpA patient journey. Participants with multiple diagnoses often described their health conditions as particularly complex and multifactorial: “When my bones are working, I can’t breathe with my asthma and when I breathe well, my bones protest.” (P3). Aside from complex health conditions, they also found navigating the healthcare system to be particularly intricate and stressful, with one participant describing it as “a whole tale of woe” (P10). This complexity often arose from integrating multiple disease-specific care paths rather than receiving holistic, coordinated care tailored to individual needs.
Overall, the interviews highlighted a desire for holistic healthcare that addresses the interplay between physical comorbidities as well as between physical and mental health: “I’m one person, it’s one journey, it’s one body with one mind, or the other way around. And it’s just intertwined.” (P14).
This study provides valuable insights into the experiences of individuals living with axSpA, contributing to a comprehensive understanding of their patient journeys and advocating for the implementation of people-centered integrated healthcare. The study reveals hurdles in the axSpA patient journey such as an unsystematic, intraspecific, and fragmented care system that requires a high level of self-responsibility. Although the diagnosis represents a turning point in which, among other things, effective medicine can be used, problems arise in connection with time-consuming treatments and social contexts. Moreover, the study reveals that the patient journey of those affected is characterized not only by their journeys through the healthcare system but also by their personal biographies. Life events, comorbidities as well as self- and external perceptions are not only considered as external influences on the axSpA journey, but rather as mutually interacting (Fig. 1 ).
Healthcare journey with axSpA
This study supports that the axSpA diagnosis is perceived by patients as a turning point in their patient journeys. However, on their journey to diagnosis, patients struggled to navigate a fragmented healthcare system. Fragmentation of healthcare systems is regarded as a downside of increasing sub-specialization 10 . In fragmented healthcare systems, health services and medical records are organized in disease-specific silos rather than streamlined care processes 15 . This affects multiple interfaces of health services, resulting in gaps between primary and secondary care, between the different medical specialties, and between medical care and psychosocial support 10 , 11 . As this study indicated, transitioning between these silos usually falls on patients themselves, requires health and navigation literacy, and often results in loss of information and time.
Before the correct diagnosis is made, people with axSpA often present with unclear back pain 1 , 4 . Contrary to the German guidelines for treating nonspecific back pain, which recommend a designated physician to coordinate diagnostic and therapeutic processes 32 , participants in this study frequently encountered symptomatic treatment for their back pain. To seek causal explanations, they had to advocate for themselves, navigate the healthcare system, and facilitate communication between specialists.
Existing care coordination initiatives, like Disease Management Programs (DMPs) are inherently standardized to specific, formally diagnosed diseases (e.g., for rheumatoid arthritis or chronic back pain), and therefore fail to streamline processes in a pre-diagnostic phase 33 . However, in accordance with previous studies 5 , 21 , 22 , 34 , this study indicated that the pre-diagnostic phase is particularly crucial in axSpA. Previous research has found a correlation between longer diagnosis delays and different non-rheumatologist visits and misdiagnoses 22 . As described by participants in this study, failing to access rheumatologic care early on may set in motion a vicious cycle where multiple specialists (general practitioners, orthopedists, and others) are consulted without achieving conclusive results. To healthcare providers, this quest for diagnosis may appear as “doctor shopping” 7 , fostering an environment of disbelief and bias that culminates in stigmatizing and psychosomatic misdiagnoses 34 .
Based on these insights, this study emphasizes the need for collaborative efforts among various non-rheumatologic professions in early detection. The growing field of eHealth holds the potential to contribute to this imperative by providing tools applicable to various stages and locations of the patient journey, from symptom checkers to self-management aids 35 . Notably, some eHealth applications are designed specifically to coordinate rheumatology care 36 . However, such applications may fall short of addressing the inherent fragmentation that hinders care processes prior to or outside of the rheumatology setting. A stronger focus of digital health should be on the integration of digital tools, e.g., through a common interface to streamline the diagnostic process and develop holistic care concepts for multimorbid patients with axSpA.
Intertwined personal and healthcare experiences in axSpA patient journeys
In addition to creating navigation hurdles and thus a higher workload for patients, fragmentation of care arguably also leads to depersonalization of healthcare where medical and personal needs are viewed separately 37 . Accordingly, previous studies have suggested to include both health events and life events in people-centered narratives of chronic illness journeys 17 , 19 , 38 .
Several studies have demonstrated the influence of individual context factors on perceptions of health 39 , 40 and healthcare 41 . This study elucidates an intricate interplay between healthcare and personal journeys. On the one hand, the study findings aligned widely with Michael Bury’s concept of ‘chronic illness as biographical disruption’ 42 , as respondents described changes in self-identity (e.g., confrontation with stigma surrounding axSpA), social networks (e.g., caregiving roles), and future perspectives (e.g., reconsidering career paths) following an axSpA diagnosis. On the other hand, biographical factors (e.g., stressful life events) also influenced the experience of axSpA. Previous studies support these findings, demonstrating bidirectional relationships between affect and inflammation markers, and affect and perceptions of pain 3 , 43 .
According to people-centered care principles, treatment should be tailored to persons rather than diagnoses 38 . Therefore, it is necessary to consider personal context factors like biographies and comorbidities that may or may not be related to axSpA. Multimorbidity is associated with a complex interplay of symptoms and treatments surpassing the sum of the individual diseases 44 . For instance, while inflammatory arthritis typically benefits from physical exercise, the presence of concomitant bronchial asthma may limit exercise capacity. Thus, the presence of non-SpA comorbidities can interfere with treatment standards for axSpA. In addition, comorbidities may impair mental well-being in people with axSpA, as suggested by an association with depressive symptoms 45 . This requires multidisciplinary disease management of axSpA beyond the rheumatology setting.
Consequently, perceptions of axSpA in social contexts, biographical factors, and interfering comorbidities can shape the trajectories of patient journeys, create complex health needs and may influence the perceived burden of axSpA. At the same time, these contextual factors are highly individual and difficult to quantify for clinical scores or to include in clinical routines. However, a more controllable perception of illness leads to more constructive health behaviors, like treatment adherence and self-care 39 , highlighting the clinical relevance of knowing the nuanced ways in which the same diagnosis can impact people’s lives differently.
Limitations
The qualitative research design of this study has inherent strengths and limitations. While the study enables nuanced and in-depth perspectives, it does not allow for representative statements about frequency distributions. Illness narratives are subjective and socioculturally constructed data sources 46 , and in this study specifically, social desirability bias may have influenced participants’ narratives due to the presence of a medical student interviewer. Although recall bias cannot be ruled out either, it can be assumed that the essential experiences were reproduced.
The contextualization of the study results may be constrained due to limited personal and demographic information about the participants. Details such as exact age, employment, or family status were kept minimal to prioritize the participants’ anonymity. Moreover, there is no indication of their exact disease durations since few participants could recall the exact onset of their symptoms and time of diagnosis, and the study primarily focused on emotional encounters and experiences rather than precisely reconstructed timelines.
Despite efforts to ensure demographic heterogeneity, the diversity and nuance of the results may be limited due to the sampling strategy (single-center recruitment) and exclusion criteria (German language, mental capacity). These criteria were selected based on the interviewer’s language proficiency (German native speaker) to facilitate clear and in-depth communication, as well as to uphold ethical standards by ensuring informed consent was based on full mental capacity, especially regarding the sensitive nature of the topic. However, this approach excludes more vulnerable or underrepresented population groups. Further research is needed to determine and implement precautions to include these valuable perspectives for a holistic, people-centered understanding.
This study presents insights in axSpA patient journeys that elicit an interplay of patients’ experiences with the healthcare system and their personal biographies. Life events, comorbidities as well as self- and external perceptions are not regarded as merely external influences on the axSpA journey, but as mutually interacting. These insights contribute to a more nuanced understanding of the axSpA patient journey, which can raise awareness for and inform people-centered care in clinical practice. The study can further contribute to future research approaches by presenting a perspective on patient journeys that extends beyond a single-disease focus and linear timelines.
Data availability
The data sets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Sieper, J. & Poddubnyy, D. Axial spondyloarthritis. Lancet 390 , 73–84 (2017).
Article PubMed Google Scholar
Garrido-Cumbrera, M. et al. The European map of axial spondyloarthritis: Capturing the patient perspective-an analysis of 2846 patients across 13 countries. Curr. Rheumatol. Rep. 21 , 19 (2019).
Article PubMed PubMed Central Google Scholar
Zhao, S. et al. The prevalence of depression in axial spondyloarthritis and its association with disease activity: A systematic review and meta-analysis. Arthritis Res. Ther. 20 , 140 (2018).
Ramiro, S. et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 82 , 19–34 (2023).
Mauro, D., Forte, G., Poddubnyy, D. & Ciccia, F. The role of early treatment in the management of axial spondyloarthritis: Challenges and opportunities. Rheumatol. Ther. 11 , 19–34 (2024).
Yi, E., Ahuja, A., Rajput, T., George, A. T. & Park, Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: A systematic review. Rheumatol. Ther. 7 , 65–87 (2020).
Lapane, K. L. et al. Primary care physician perspectives on barriers to diagnosing axial Spondyloarthritis: A qualitative study. BMC Fam. Pract. 21 , 204 (2020).
Danve, A. & Deodhar, A. Axial spondyloarthritis in the USA: Diagnostic challenges and missed opportunities. Clin. Rheumatol. 38 , 625–634 (2019).
Gudu, T. & Jadon, D. R. Multidisciplinary working in the management of axial and peripheral spondyloarthritis. Ther. Adv. Musculoskelet. Dis. 12 , 1759720X20975888 (2020).
Sofaer, S. Navigating poorly charted territory: Patient dilemmas in health care ‘nonsystems’. Med. Care Res. Rev. 66 , 75–93 (2009).
Article ADS Google Scholar
Nolte, E. et al. Overcoming fragmentation in health care: Chronic care in Austria, Germany and the Netherlands. Health Econ., Policy Law 7 , 125–146 (2012).
World Health Organization, World Bank Group, & OECD. Delivering Quality Health Services: A Global Imperative for Universal Health Coverage (2018). Available at https://doi.org/10.1596/978-92-4-151390-6 .
Joseph, A. L., Monkman, H., Kushniruk, A. & Quintana, Y. Exploring patient journey mapping and the learning health system: Scoping review. JMIR Hum. Factors 10 , e43966 (2023).
Davies, E. L. et al. Reporting and conducting patient journey mapping research in healthcare: A scoping review. J. Adv. Nurs. 79 , 83–100 (2023).
Halvorsrud, R., Lillegaard, A. L., Røhne, M. & Jensen, A. M. Managing complex patient journeys in healthcare. In Service Design and Service Thinking in Healthcare and Hospital Management: Theory, Concepts, Practice (eds Pfannstiel, M. A. & Rasche, C.) 329–346 (Springer, 2019).
Chapter Google Scholar
Momen, N., Kendall, M., Barclay, S. & Murray, S. Using timelines to depict patient journeys: A development for research methods and clinical care review. Prim. Health Care Res. Dev. 14 , 403–408 (2013).
Salamonsen, A., Kiil, M. A., Kristoffersen, A. E., Stub, T. & Berntsen, G. R. “My cancer is not my deepest concern”: Life course disruption influencing patient pathways and health care needs among persons living with colorectal cancer. Patient Prefer. Adherence 10 , 1591–1600 (2016).
Rix, E. F., Barclay, L., Stirling, J., Tong, A. & Wilson, S. ‘Beats the alternative but it messes up your life’: Aboriginal people’s experience of haemodialysis in rural Australia. BMJ Open 4 , e005945 (2014).
Sezgin, E., Weiler, M., Weiler, A., Lin, S. & Hart, L. It is a life journey: A roadmap of teens with chronic diseases in transitioning to independence. J. Pediatr. Health Care 34 , 346–355 (2020).
Wilson, N. et al. Exploring the emotional impact of axial Spondyloarthritis: A systematic review and thematic synthesis of qualitative studies and a review of social media. BMC Rheumatol. 7 , 26 (2023).
Martindale, J. & Goodacre, L. The journey to diagnosis in AS/axial SpA: The impact of delay. Musculoskelet. Care 12 , 221–231 (2014).
Article CAS Google Scholar
Ogdie, A. et al. Real-world patient experience on the path to diagnosis of ankylosing spondylitis. Rheumatol. Ther. 6 , 255–267 (2019).
Tong, A., Sainsbury, P. & Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 19 , 349–357 (2007).
Saunders, B. et al. Saturation in qualitative research: Exploring its conceptualization and operationalization. Qual. Quant. 52 , 1893–1907 (2018).
Helfferich, C. Leitfaden- und Experteninterviews. In Handbuch Methoden der empirischen Sozialforschung (eds Baur, N. & Blasius, J.) 875–892 (Springer, 2022).
Otón, T., Sastre, C. & Carmona, L. The journey of the non-radiographic axial spondyloarthritis patient: The perspective of professionals and patients. Clin. Rheumatol. 40 , 591–600 (2021).
WebMD Ignite. Patient Journey Mapping FAQ. Available at https://webmdignite.com/faq/what-is-patient-journey-mapping .
Wilms, A. RED - Sichere Lösungen für das Gesundheitswesen. Available at https://www.redmedical.de/ .
Krouwel, M., Jolly, K. & Greenfield, S. Comparing skype (video calling) and in-person qualitative interview modes in a study of people with irritable bowel syndrome–an exploratory comparative analysis. BMC Med. Res. Methodol. 19 , 219 (2019).
Kuckartz, U. Qualitative Inhaltsanalyse: Methoden, Praxis, Computerunterstützung (Beltz Juventa, 2012).
Google Scholar
Benson, M. et al. Development of a patient journey map for people living with cervical dystonia. Orphanet J. Rare Dis. 17 , 130 (2022).
Chenot, J. F. & Becker, A. Update der Nationalen Versorgungsleitlinie Kreuzschmerz. Z. Allg. Med. 93 , 250–254 (2017).
Gemeinsamer Bundesausschuss. Disease-Management-Programme. Available at https://www.g-ba.de/themen/disease-management-programme/ .
Dube, C. E. et al. Personal experiences with diagnostic delay among axial spondyloarthritis patients: A qualitative study. Rheumatol. Ther. 8 , 1015–1030 (2021).
Granström, E., Wannheden, C., Brommels, M., Hvitfeldt, H. & Nyström, M. E. Digital tools as promoters for person-centered care practices in chronic care? Healthcare professionals’ experiences from rheumatology care. BMC Health Serv. Res. 20 , 1108 (2020).
Welcker, M. et al. Digitale Tools entlang der Patient Journey. Available at https://www.bdrh-service.de/wp-content/uploads/2022/01/eBooklet_RheumaIT.pdf .
Stange, K. C. The problem of fragmentation and the need for integrative solutions. Ann. Fam. Med. 7 , 100–103 (2009).
Hansen, F., Berntsen, G. K. R. & Salamonsen, A. “What matters to you?” A longitudinal qualitative study of Norwegian patients’ perspectives on their pathways with colorectal cancer. Int. J. Qual. Stud. Health Well-being 13 , 1548240 (2018).
Petrie, K. & Weinman, J. Why illness perceptions matter. Clin. Med. 6 , 536–539 (2006).
Article Google Scholar
Arat, S., De Cock, D., Moons, P., Vandenberghe, J. & Westhovens, R. Modifiable correlates of illness perceptions in adults with chronic somatic conditions: A systematic review. Res. Nurs. Health 41 , 173–184 (2018).
Sofaer, S. & Firminger, K. Patient perceptions of the quality of health services. Annu. Rev. Public Health 26 , 513–559 (2005).
Bury, M. Chronic illness as biographical disruption. Sociol. Health Illn. 4 , 167–182 (1982).
Article CAS PubMed Google Scholar
Carroll, J. E. et al. negative affective responses to a speech task predict changes in interleukin(IL)-6. Brain Behav. Immun. 25 , 232–238 (2011).
Duguay, C., Gallagher, F. & Fortin, M. The experience of adults with multimorbidity: A qualitative study. J. Comorb. 4 , 11–21 (2014).
Redeker, I. et al. Determinants of psychological well-being in axial spondyloarthritis: An analysis based on linked claims and patient-reported survey data. Ann. Rheum. Dis. 77 , 1017–1024 (2018).
Bury, M. Illness narratives: Fact or fiction?. Sociol. Health & Illn. 23 , 263–285 (2001).
Download references
Acknowledgements
We express our gratitude to the participants of this study for sharing valuable insights into their patient journey experiences. We also sincerely thank Maximilian Schielein for contributing to the conception and design of this study. Additionally, we acknowledge the use of Large Language Models for translation and grammar checking in non-sensitive sections of the manuscript and note that Fig. 1 was created by the authors in Lucid (lucid.co).
Open Access funding enabled and organized by Projekt DEAL. This manuscript was funded by the Department of Dermatology and Allergy of the Technical University of Munich and financially supported by Novartis Pharma GmbH.
Author information
Authors and affiliations.
Department of Dermatology and Allergy, TUM School of Medicine and Health, Technical University of Munich, Biedersteiner Str. 29, 80802, Munich, Germany
Kristina Berr, Stefanie Ziehfreund, Tilo Biedermann & Alexander Zink
Medizinisches Versorgungszentrum für Rheumatologie Dr. M. Welcker GmbH, Planegg, Germany
Martin Welcker
Division of Dermatology and Venereology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden
Alexander Zink
You can also search for this author in PubMed Google Scholar
Contributions
Study conception and design: K.B., S.Z., M.W., T.B., A.Z. Data acquisition: K.B., S.Z., M.W., A.Z. Analysis and interpretation: K.B., S.Z., A.Z. K.B. and S.Z. took the lead in writing the manuscript. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Corresponding author
Correspondence to Alexander Zink .
Ethics declarations
Competing interests.
AZ has been an advisor and/or received speaker’s honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Almirall, Amgen, Beiersdorf Dermo Medical, Bencard Allergie, BMS, Celgene, Eli Lilly, GSK, Janssen Cilag, Leo Pharma, Miltenyi Biotec, Novartis, Pfizer, Sanofi-Aventis, Takeda Pharma, UCB Pharma. KB, SZ, MW and TB declared no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary table s1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Berr, K., Ziehfreund, S., Welcker, M. et al. A qualitative exploration of the patient journey in axial spondyloarthritis towards a people-centered understanding. Sci Rep 14 , 19977 (2024). https://doi.org/10.1038/s41598-024-70420-8
Download citation
Received : 22 May 2024
Accepted : 16 August 2024
Published : 28 August 2024
DOI : https://doi.org/10.1038/s41598-024-70420-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Axial spondyloarthritis
- Patient journey
- Qualitative research
- People-centered care
- Integrated care
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Supporting Novartis with their pre-launch strategy

Giving a first-in-class cardiovascular treatment a best-in-class launch strategy
“This project has set the gold standard for how patient journey research should be done for all future launch products. We were able to use the project outputs to brief the Launch Team on expected challenges with the launch and propose solutions that could be built into our operational plan, in a timely manner.”
UK product lead, Novartis
A comprehensive and iterative approach to support Novartis with the development of launch operation plans and tactics.
An exciting opportunity with lots of unknowns
Novartis is expanding its portfolio in the cardiovascular space with a first-in-class treatment.
As well as being a novel product offering, the intended prescribing customer was new to our client.
No stone left unturned
The preparatory stage set the project up for success; we spent time defining the business challenge, generating ongoing hypotheses and collaborating with other partner agencies to ensure a seamless approach to data insight collection, triangulation and consolidation.
Our comprehensive, multipronged and iterative approach provided a 360◦ view on the prescribing pathway and the network of influencing stakeholders.
Delivering focus and action for Novartis
The outputs were insight and recommendation-led, focused on highlighting key areas of opportunity ie stakeholders most relevant for the novel product launch, in addition to potential pain points in the patient pathway ie patients lost in follow-up.
This helped to shape operational plans for Novartis and specifically:
- Provided a comprehensive briefing for the launch brand team
- Supported the task force decisions making
- Fed directly into tactical planning
This research was highly commended by BHBIA .

“This project has provided invaluable insights that has allowed us to better understand the current cardiovascular space in the UK, informing the planning and decision making within the launch team. The output is a fantastic resource, consolidating not only the insights from the commissioned research itself but also pivotal data from a tandem research project and other internal market research.”
UK Business Analytics and Insights Manager, Novartis
Read more from our Health team here .
Healthcare , Patient centricity , Planning , Research , Strategy

How Novartis deploys a new model of creativity to understand patients better
International Journal of Pharmaceutical and Healthcare Marketing
ISSN : 1750-6123
Article publication date: 26 June 2023
Issue publication date: 25 July 2023
To better understand the reality of living with the diseases and conditions that its drugs and therapies are developed to treat, the Novartis leadership determined a need for more meaningful insights into patients’ lives. They sought to develop a systematic, creative methodology – informed by the psychology of insightful rather than analytical thinking – to properly integrate and deploy the research commissioned into its day-to-day business decision-making. For it is well established that better understanding of the patient reality drives both compliance and adherence “beyond the pill”. The purpose of this paper is to bring the novel methodology of creativity to a wider audience and ensure that many others – notably in patient advocacy organizations – can benefit from this approach.
Design/methodology/approach
A core team of Insight and Analytics and Patient Engagement leads from various therapeutic area teams worked in partnership with a psychologist and practitioner in the field of insightful thinking, to develop an effective methodology that could reliably surface and articulate genuine patient insights. This methodology – the i4i Insights Discovery™ process – was developed, piloted, refined and codified in 2020 and implemented across the company in 2021–2022. It uses a combination of convergent and divergent thinking techniques – human rather than artificial intelligence, combining diverse research outputs – to understand patients’ lives better. With enhanced understanding, the insights then shape educational and behavioral strategies to drive adherence and compliance.
At a time of tightening budgets and demands to deliver enhanced impact from research budgets, i4i Insights Discovery™ has enabled Novartis teams to turn existing research outputs into profound and useful understandings of what it means to live with specific diseases and develop evidence-based patient engagement strategies; insight-driven decision-making around the lifecycle of any compound. i4i Insights Discovery™ has been applied across Novartis’s diverse areas of expertise, from heart disease to cancer, from organ transplantation to dermatology, from food allergy to ophthalmology.
Practical implications
The i4i Insights Discovery™ process enables Novartis teams to gain deeper understanding of patients’ lives without the need to commission additional research; to do more with less. These insights enable cross-functional Novartis teams to develop better-informed strategies that better address the needs of patients and their care partners, of health-care professionals and health-care systems. The team creating the process is looking to make the i4i Insights Discovery™ approach a gold standard of insight discovery, both for pharma and health care and in other categories, too.
Originality/value
The i4i Insights Discovery™ process is a practical, novel application of well-established principles in the psychology of insightful thinking to address a clear business imperative. By repurposing and reinterpreting existing research outputs using creative verbal and visual exercises, it delivers a more human and empathetic understanding of the patient reality. It moves teams from “So what?” – this is what the data mean – to “Now what?” – this is what we should do as a result.
Knowles, S.K.Z. and Klein, B. (2023), "How Novartis deploys a new model of creativity to understand patients better", International Journal of Pharmaceutical and Healthcare Marketing , Vol. 17 No. 3, pp. 311-326. https://doi.org/10.1108/IJPHM-11-2022-0100
Emerald Publishing Limited
Copyright © 2023, Samuel Kenneth Zachary Knowles and Beyza Klein.
Published by Emerald Publishing Limited. This article is published under the Creative Commons Attribution (CC BY 4.0) licence. Anyone may reproduce, distribute, translate and create derivative works of this article (for both commercial & non-commercial purposes), subject to full attribution to the original publication and authors. The full terms of this licence may be seen at http://creativecommons.org/licences/by/4.0/legalcode
Introduction
There are many factors affecting whether or not patients comply with the recommendations of their physicians and adhere to the medications prescribed to address the diseases or conditions from which they suffer. A major meta-analysis of the extent and determinants of non-compliance ( Morris and Schulz, 1992 ) found that adherence and compliance – two critical factors that ensure drugs have the best chance to work as intended – can be improved through a combination of both educational and behavioural strategies, and a comprehensive understanding of what it is like to suffer from specific conditions and live with the reality of the medications prescribed.
Non-compliance and lack of adherence occurs among patients with diseases and conditions that are both acute (requiring time-limited medication) and chronic (requiring medication to be taken for the rest of the patient’s life; Zisook and Gammon, 1981 ). This includes medications deemed to be vital for both quality of life and the prolongation of life expectancy, including drugs that regulate life-curtailing, chronic diseases such as cardiovascular disease ( Joshi et al. , 1999 ), cancer ( Kostev et al. , 2014 ) and even following organ transplantation ( Laederach-Hoffman and Bunzel, 2000 ).
At face value, the decision of patients not to take medications can appear to be counter-intuitive – particularly to physicians and researchers in pharmaceutical companies, who understand the relative and absolute risk for patients with specific diseases and conditions and their medication regimens. Reasons for non-compliance and failure to adhere to a regimen of medication – particularly multiple medications required to manage one or several diseases or conditions – are many and various. They can include: acute or chronic side effects, fear, worry, depression, cost, incompatibility with lifestyle, psychological denial, a lack of symptoms (not feeling ill), a mistaken belief that a chronic disease or condition has been controlled or cured by the acute administration of medication, mistrust of medicine in general and forgetfulness ( AMA, 2020 ).
Designing strategies that improve health outcomes for patient communities – educational and behavioural interventions that go “beyond the pill” – is an imperative for innovation and success across the pharmaceutical industry ( Wenzel and Henne, 2014 ). For these initiatives to succeed, however, it is vital that those developing them have meaningful, disease-specific insights into the lives of patients – and often the lived experience of care partners of those patients, too. This is particularly true for care partners of those suffering from neurological and neurodegenerative conditions ( Pollock, 2021 ), as well as care partners (often parents or guardians) of minors ( Rotberg et al. , 2020 ). Both categories of care partners can play a significant – if not total – role in patient decision-making around their treatment.
To enhance patient adherence and compliance and at the same time improve patient knowledge – to develop medicines and supportive patient engagement programs that meet patient needs and so improve long-term health outcomes – in 2019 Novartis’s leaders identified the need to standardize and systematize the company’s determination to seek out and unlock insights from patient communities. To increase patient focus, they saw that they needed to bring the voice, experience and perspective of patients to the heart of drug development, design and delivery.
Beyond data, information and observations of patients’ lives, Novartis started out on a journey to create an effective way of bringing patient insights to the heart of decision-making. This required the development of a creative thinking process in an analytical thinking culture – two fundamentally different types of cognition. Additionally, the process was architected to enable Novartis teams to surface and articulate insights into other stakeholder groups too, including care partners and health-care professionals. It was stakeholder-agnostic, by design.
Aims and structure of this paper
The aims of this paper are to explore and explain how Novartis developed its i4i Insights Discovery™ process. By detailing how and why the process was developed, piloted, refined, codified and rolled out across the company as the “by Novartis, for Novartis” methodology for surfacing and articulating insights, the paper aims to make this approach a gold standard in insight discovery. It is our intention to establish i4i Insights Discovery™ as a way of thinking and working with existing research assets that can benefit others – in the pharmaceutical as well as other sectors.
The methodology described enables Novartis teams to make the research studies they commission work harder for them – and for the patients they serve. By synthesizing the findings from a wide variety of research inputs – primary and secondary market research, patient segmentation, social media listening reporting, academic papers and patient advisory boards – the process enables cross-functional teams to surface and articulate meaningful insights into the lives of patients and care partners in any disease or condition. It overlays human intelligence and creativity on data and research outputs that too-often are not turned into meaningful, patient-facing activities.
By understanding patients and their perspectives better, teams then use their empathetic understanding of the patient reality to develop therapeutic area strategies, educational and behavioural interventions, that better meet patient needs. This, in turn, has the potential to improve patient health outcomes thanks to greater adherence and compliance to drugs and other therapies proven to be effective in clinical trials.
This paper covers the development of the research methodology, its impact and the implications this way of thinking and working has had to date – and can have in the future. It considers the implications for practitioners and the wider research community, while also highlighting the limitations of the process and ways in which it could be enhanced.
Research methodology
Advanced research tools and techniques are fundamental to how Novartis seeks to understand the patient experience and so deliver therapies that are compatible with patients’ lives. These include primary market research, social media listening, market intelligence tracking and collaborating with patient organizations. Novartis’s 700-strong Insight and Analytics team in Hyderabad is one of the largest pharma research communities anywhere in the world. Neither the Hyderabad team nor the Patient Engagement leads in different franchise areas lacked data. It was time to establish how to move from data to actionable insights that help reimagine medicine.
In 2019, Novartis’s leaders identified the need to standardize and systematize the company’s determination to seek out and unlock patient insights, while of course respecting all applicable confidentiality and data privacy requirements. The first step was to establish a senior project team with active collaboration across functions – Insights and Analytics, Patient Engagement, Clinical and Medical. This core team also enjoyed representation across the medicines’ lifecycle, from the Novartis Institute of Biomedical Research, Global Drug Development and all the company’s franchises, focused on specific disease areas. The team was cross-functional by design because it is well established that diverse teams whose members have different experience, backgrounds and areas of expertise are more likely to develop more diverse, more creative and fundamentally more insightful outputs ( Jansen and Searle, 2021 ; De Bono, 1985 ).
The six-strong team – which together had more than 100 years’ experience in insight, innovation and patient engagement roles, in both pharmaceutical marketing and consumer goods – pooled all the resources, tools and methodologies for surfacing and articulating insights that they had used productively during their careers. This included several different variants of the Design Thinking approach pioneered by David Kelley at Stanford University’s d.school and in the consultancy IDEO ( Kelley, 2001 ), Edward De Bono’s “Six Thinking Hats” methodology ( De Bono, 1985 ) and the process created by Allan and collaborators in the creative consultancy? WhatIf! ( Allan et al. , 1999 ).
The Novartis team also sought the external perspective of an independent expert in the field of insight and insightful thinking to be its partner in developing its own, proprietary methodology for surfacing and articulating insights. They engaged psychologist Dr Sam Knowles, a 30-year veteran of the analytics and insight industry, author of the book How To Be Insightful: Unlocking the Superpower that Drives Innovation ( Knowles, 2020 ) and creator of the STEP Prism of InsightTM, a model designed to systematically surface and articulate insights.
Setting a high-bar definition of insight
The explosion of data in the past 20 years means that all categories of business – and all functions within businesses – are now data-rich ( Mayer-Schonberger and Cukier, 2013 ), with more information and metrics at their disposal than ever before. The challenge most people in the modern knowledge economy face is not data poverty, it is data excess or infobesity ( Knowles, 2018 ). As a consequence, the term and the concept of insight has become devalued ( Berinato, 2019 ). Too often, data points, information and casual observations are presented and accepted as “insights” but they do not satisfy even a dictionary definition of the word; the Cambridge Dictionary defines insight as: “a clear, deep, and sometimes sudden understanding of a complicated problem or situation, or the ability to have such an understanding”.
Accordingly, as a first step in developing a proprietary process for turning information into insight, the team set a high-bar definition of insight to become the Novartis understanding of the concept. They settled on the following:
A profound and useful understanding of our customers’ attitudes, behaviors, or beliefs that enables us to reimagine actions and so establish a deeper connection and relevance between us and their lives.
The definition was designed to be challenging, aspirational and directive. By moving beyond data, information and casual observations, it aims to help those working with it answer the questions “So what?” – “What do the data mean?” – and, armed with that understanding, move on to answering the practical questions “Now what?” – “What can we do with that understanding to help those we are working with?”. In the context of Novartis, this means understanding what it is like for patients (and other stakeholders, including care partners and health-care professionals) to live with the diseases and conditions that affect them. By better understanding the patient reality and issues that get in the way of adherence and compliance to prescribed treatments, relevant teams could design educational and behavioral strategies rooted in patients’ lived experience. As a result of this deeper connection, because these strategies build in the patient perspective, the hypothesis is that they are also more likely to succeed than those that do not.
Insightful thinking in an analytical thinking culture
The pharmaceutical and health-care sector is primarily an analytical thinking culture. Evidence, proof and science are demanded as a fundamental cornerstone and underpinning of all problem-solving and decision-making in the development and marketing of novel, effective and safe drugs and therapies for different diseases and conditions.
The challenge in developing a systematized process for insightful thinking is that insightful thinking is not the same as analytical thinking ( Knowles, 2020 ). Analytical thinking yields to time input, intellect and brain power; it is linear and logical and reducible to predictable – if often complex – formulae; it thrives on time spent working on the challenge thanks to the sustained application of conscious processing. Insightful thinking, meanwhile, involves the recombination of existing knowledge in new and hitherto untried ways; thrives on distraction and timeout; requires the focused application of subconscious processing; and delivers immediate comprehension ( Kounios and Beeman, 2015 ).
The psychology of problem-solving is dominated by two complementary cognitive models: representational change theory and progress monitoring theory. Representational change theory ( Knoblich et al. , 1999 ) posits that to solve a problem, we must deconstruct it into its component parts and use a combination of logic and long-term memory (past experience) to resolve them. While this works efficiently in solving analytical problems, in insightful thinking we are looking to join together existing information in new and hitherto untried ways. The strategy that works for analytical thinking problems turns out not to work for insightful thinking problems because of the constraints or impasses that prior knowledge puts in the way.
Progress monitoring theory ( MacGregor et al. , 2001 ) suggests that when we are trying to solve a problem, we monitor how we are getting on with the task at hand, decompose it into chunks and assess progress based on performance on the component parts. We are only satisfied that we have solved the problem when our progress monitoring suggests all chunks have been satisfactorily addressed. Again, because prior experience and knowledge introduce constraints or impasses, when faced with the challenge of surfacing and articulating insights, we find our usual, analytical problem-solving strategy unhelpful.
A technique for producing ideas
Preparation: the problem is investigated from all directions.
Incubation: not consciously thinking about the problem.
Illumination: the appearance of the happy idea.
Verification: the validity of the idea is tested.
This same, four-step approach was described independently in many different fields, from the late 19th to the mid-20th centuries, from music to biology, from mathematics to novel writing, from chemistry to advertising ( Flesch, 1951 ). It features in John Livingston Lowes’ monumental study of how Coleridge created his poem, Kubla Khan ( Lowes, 1927 ), in advertising executive James Webb Young’s A Technique for Producing Ideas ( Webb Young, 1940 ) and in studies of creativity in engineers ( Cropley, 2016 ).
To join existing knowledge, data and understanding together in new and unexpected ways, we need to relax the analytical constraints on our thinking, making it – in the words of Leonard Mlodinow, the Caltech physicist who helped to resolve the psychology of insightful thinking – more elastic and flexible ( Mlodinow, 2018 ). This means that we need to throw off the usual shackles and constraints of analytical thinking.
In part, this is achieved by total immersion in the subject matter area. In part it requires deliberately building timeout into the creative process. And in part it demands those looking to surface and articulate insights use a combination of convergent and divergent thinking techniques to foster insight generation (following Guildford, 1959 ; see practical applications in engineering in Cropley, 2016 ). Divergent thinking techniques open up possibilities and options, introducing new data; convergent thinking techniques force us to make choices and discard data, focussing on what matters. What psychology has demonstrated experimentally has also recently been confirmed by both positon emission tomography (PET) and functional magnetic resonance imaging (fMRI) brain-scanning technology ( Kounios and Beeman, 2015 ). For example, research in Kounios and Beeman’s labs show that, just before we solve an insight problem, our visual cortex – about 40% of our brains – effectively shuts down. The floodlight of attention becomes a laser-focused spotlight, presaging – in Wallas’s words – “the appearance of the happy idea”.
Aligning the planets: creating a systematized process for insightful thinking
Guided by these principles rooted in the psychology and neuroscience of insightful thinking, the cross-functional Novartis working party developed a systematized, creative, insightful thinking process. The process was designed to enable teams working across the business to surface and articulate genuine patient insights and so better understand the patient – and care partner – reality of living with any and all of the diseases and conditions its drugs and therapies exist to treat. In this way, the methodology was an insightful thinking process specifically designed to work inside what always has been – and always will be – a predominantly analytical thinking culture.
By taking any and all research outputs the company commissions, the process sought to enable its teams to do more with less; to spot commonalities and alignments in data from primary market research, social media listening, patient segmentation and other research outputs and craft these into genuine insights into patients’ lives. In this way, the company aimed to institutionalize patient insight-driven decision-making, enhance adherence and compliance and achieve its mission of improving health outcomes.
The i4i Insights Discovery™ process was designed as a four-stage methodology run in what were designated as Insight Sprints, each stage named after one of the four “I”s of insightful thinking – IMAGINE, IMMERSE, INTEGRATE and INSPIRE. These four stages were in part inspired by the four-step models developed independently in many domains during the 20th century ( Wallas, 1926 ; Flesch, 1951 ), although the four i4i Insights Discovery™ stages do not map perfectly onto those earlier models. Insight Sprints are designed to run over a four-to-eight-week period, with up to 12 participants required to dedicate up to three full days of their time to the process while they continue to fulfil their responsibilities within Novartis. This time comprises multiple creative and action-planning workshops, information gathering and preparation and immersive patient experiences.
Throughout the i4i Insights Discovery™ process, convergent and divergent thinking exercises are conducted in a similar way, underpinned by the principle that diverse minds with diverse experience and different perspectives are more likely to generate a richer understanding of the patient reality ( De Bono, 1985 ). Up to 12 participants come together from different functions and disciplines from within Novartis, including patient engagement, clinical development, medical affairs, market access, marketing, commercial, regulatory affairs, health economics, insight and analytics, new product development and innovation and pipeline strategy. For the creative thinking exercises that characterize the process, all participants are briefed together as a group. They then split into breakout rooms – real or virtual – and work alone initially, then share their creative outputs in small groups of three to five. They blend and merge ideas together in these groups, and then bring their integrated outputs back to the whole team, where ideas are shared in plenary. In this way, diverse experience and perspectives are continually heard and blended, with no one voice dominating.
IMAGINE – articulating the key challenge for which insights are required
Insights do not exist in a vacuum, and understanding the patient reality needs focus. This is why the first workshop in i4i Insights Discovery™ introduces all participants to the process, explains the importance and consequences of the high-bar definition of insight (above) and has them create a key challenge for the Insight Sprint for which insights are required. Examples of great insights that have effected change – from outside as well as inside the pharmaceutical and healthcare business – are shared by way of stimulus. Additionally, the principles of asking smarter questions are detailed ( Knowles, 2022 ), together with examples of how key challenges have been framed in previous Insight Sprints.i4i Insights Discovery™ sought inspiration from a variety of different creative thinking methodologies, including several different variants of the Design Thinking approach ( Kelley, 2001 ). In its Design Sprints, Design Thinking typically uses a question that starts with the inclusive formula “How might we […]?”, and this is typically the way in which key challenges in an i4i Insights Discovery™ Insight Sprint are framed.
The principal output of the IMAGINE stage is a clear and agreed articulation of the key challenge for which the cross-functional team needs insights. Key challenges are framed according to the following principles, in that they: are open not closed questions; avoid technical, scientific or over-medicalized language; open up multiple possible responses; consider life and life experience from the patient’s perspective; are designed to elicit stories, human truths and empathy; and are answers with solutions that are more complex and nuanced than simply recommending the use of products, treatments or therapies. With the key challenge set, the process enters the second stage, IMMERSE.
IMMERSE – bringing all participants to the same level of informed knowledge
Something cannot come from nothing. To foster insightful thinking – to facilitate the joining together of “old and old to make something new” – individuals and teams need to have an established base of knowledge. In many corporate cultures, information is shared in the form of colleagues making presentations to one-another. Yet it is well-established that the “death by PowerPoint” approach is counter-productive to creativity ( Amabile et al. , 2017 ; Roberts, 2018 ; Moore, 2007 ). Indeed, we learn and acquire knowledge best and process it deeper when we adopt a “learning by doing” approach ( Reese, 2011 ; Felder and Brent, 2003 ), a principle advocated from Plato onwards, and endorsed with increasing evidence by thinkers as diverse as Marx, Montessori, Watson and Skinner.
Accordingly, to bring all participants to the same level of informed knowledge, the IMMERSE stage of the i4i Insights Discovery™ process does not involve hours of presentations but instead provides a journey of self-discovery. The Insight and Analytics and Patient Engagement leads in the Insight Sprint curate existing evidence and shares this in summary form. Participants are encouraged to explore research reports in full when specific data triggers their interest. The guided process of discovery is designed to take up to four hours across two weeks, with a summary and all original research debrief documents shared on a Microsoft Teams space.
In addition, for each Insight Sprint an immersive patient experience is developed for all participants to undertake. At its most basic, this involves watching live or recorded interviews with care partners, patient advocates and patients of the disease or condition in question. In the case of common conditions – for instance, presbyopia; age-related long-sightedness, which affects four in five people aged 50 or over – participants are provided with a discussion guide to ask friends and family what it is like living with the condition. For other conditions – such as food allergy – participants are given guidelines on living for a day “as if” they suffered themselves and then given the opportunity to write a short, reflective essay on how they found it. Another approach adopted successfully is for participants to live a structured, simulated “day in the life” of a patient, interacting with actors and digital artefacts to experience some aspects of life with the disease or condition.
The IMMERSE stage typically lasts around two weeks and is designed to fit around participants’ day jobs. They record the experience of immersion in the patient reality of the disease or condition – mediated via guided readings, videos and the immersive experience – in a bespoke workbook in which they capture the most promising emerging themes. Participants bring their completed workbooks with them to the creative workshops at the heart of the process, INTEGRATE.
INTEGRATE – turning data and information into insights
The third stage of the process is known as INTEGRATE, and it is the beating, creative heart of i4i Insights Discovery™. INTEGRATE typically runs over three, half-day sessions. The workshop assignments alternate between convergent and divergent thinking exercises, enabling individuals, small groups and the whole team to flex between making choices and creating options; to zoom in and zoom out, as they come to share and understand the patient reality better. There are up to ten exercises run during the course of the INTEGRATE stage.
This stage is split over three, half-day workshops, typically run over a week or two. This is done for three reasons. First, to maximize creative output and minimize burnout. Second, to ensure that the process does not intrude excessively on participants’ everyday responsibilities. And third, to allow meaningful periods of timeout between the sessions to allow participants’ subconscious minds to combine and recombine the information and data they are exposed to in new ways. For not only do participants learn new evidence and proof points from one another; the creative, storytelling exercises that they all undertake and share with each other also provide further stimulus and fresh perspectives to enrich and enhance their own creativity (after Storr, 2020 ).
Example divergent thinking exercise: “Adjectives, Verbs, Nouns”
An example of a divergent thinking exercise run during INTEGRATE is a task called “Adjectives, Verbs, Nouns” ( Knowles, 2022 ). Adjectives connote emotion, verbs action and nouns facts. Analysis of the language that pharmaceutical and health-care companies use to describe what they do, how and why shows that – if just adjectives, verbs and nouns are counted – the split is typically 70% nouns, 15%–20% verbs and just 10%–15% adjectives. This imbalance shows how most pharma businesses talk to those they are looking to influence rationally. Yet the psychology of decision-making shows that we make our decisions emotionally, not rationally, using the non-verbal, evolutionarily ancient reptilian and limbic brain ( Kahneman, 2011 ). We only go on to justify them rationally, using evidence, data and facts once decisions have been made.
This exercise seeks to address this imbalance and generate new expressions of the patient reality. First, small groups of up to five participants spend ten minutes generating a long list of the most distinctive adjectives describing the disease or condition which is the focus of the Insight Sprint. They then spend the same period generating lists of distinctive verbs and then nouns. Finally, participants take it in turn to compose novel sentences using at least one of the adjectives, verbs and nouns in the long lists. By addressing the adjective–verb–noun imbalance typically found in pharma communication, it is more likely that the novel sentences will contain more emotion and be real stories about real people really experiencing something. These are often the beginnings of stories that are triggered by genuine insights into patients’ lives – a profound and useful understanding of what it means to live with and manage their disease or condition.
Example convergent thinking exercise: “The Pixar Pitch”
An example of a convergent thinking exercise run during INTEGRATE is a task known as the Pixar Pitch. Disney’s Pixar animation studio is one of the world’s finest storytellers. Before any narrative is even considered for storyboarding and ultimately production by Pixar, every single story has to be expressed in a simple, six-cell formula ( Catmull, 2014 ; Pink, 2014 ):
“Once upon a time […]”
“Every day […]”
“Then one day […]”
“Because of that […]”
“Until finally […]”
In this exercise, participants are required to use this template to write a patient-focused story using the Pixar template. It might be based on one of the most promising emerging themes identified during the IMMERSE stage, captured in participants’ pre-workshop workbooks. It might be based on one of the emerging themes that others picked out. It might be an extension or an expression of the patient reality they or other participants have described in one of the other creative exercises. Most likely, it will be a blend of several different sources. It is up to participants to write the story that they feel best represents a key aspect of the patient reality. That’s the point of INTEGRATE: bringing together multiple sources of understanding to gain meaningful insights into patients’ lives.
Crafting patient insights
There are many different ways – and no definitive, single best way – to express insight. Because of the high-bar definition of insight as “a profound and useful understanding” – so much more than simply data, information, or casual observations – the format participants use in i4i Insights Discovery™ comprises two causally-connected statements and a consequence, thus:
[STATEMENT 1] […] because of […] [STATEMENT 2] […] which means […] [CONSEQUENCE].
The causal connectivity between “old and old to make something new” has already been established in earlier creative exercises, including the Pixar Pitch described above (“Because of that […]” and “Because of that […]”). In the final half-day workshop of the INTEGRATE stage of the process, participants articulate insights using this formula. Throughout the INTEGRATE stage, the workshop facilitator shares with participants examples of insights expressed using this formula – from outside and inside the pharma and health-care industry, including in previous i4i Insights Discovery™ Insight Sprints. By the time they come to write insights themselves, they are already familiar with the format.
Participants write insights individually and share them in small groups. These groups bring forward the insights they believe best address the key challenge to all participants in plenary. After the end of the third workshop, the facilitator clusters insights into themes. Typically, an i4i Insights Discovery™ Insight Sprint generates up to 50 different insights and they cluster into four-to-six themes. The facilitator shares these with all participants before the final, action-planning workshop called INSPIRE.

INSPIRE – turning insights into action
The INSPIRE workshop typically runs up to a week after the INTEGRATE workshops. At the start of INSPIRE, all insights generated during INTEGRATE are reviewed and discussed. Participants then vote – using an app, in secret, so that the loudest or most persuasive voice does not sway opinion unfairly under so-called “group think” conditions ( Janis, 1972 ) – on which clusters (or themes) of insights best address the key challenge set in the first, IMAGINE workshop. Using these themes and insights as their inspiration, individuals in small groups then rapidly develop a series of high-impact action plans. The small groups refine (and often merge, because of similarities) individual action plans, then share these with the whole group in plenary. The whole group maps these action plans onto a 2 × 2 matrix of implementation feasibility by potential impact. The group chooses the three action plans it believes are most promising and best address the key challenge, committing to bring them to life over the year ahead. Often, these data-driven, insight-rich action plans form part of other, established planning processes for – say – patient engagement already under way inside Novartis.
Piloting, refinement, codification and Cascade
The core team developed the i4i Insights Discovery™ methodology during 2020 and piloted it with a global team working in Novartis’s ophthalmology franchise. The experience of the pilot led to refinements in the process – of individual exercises, of the flow of exercises within stages, and of the process as a whole. Following these refinements, the team codified the process end-to-end in a comprehensive User Guide, as well as developing all the materials required to facilitate the process, such as slide decks to run each stage and every workshop, exercise templates and examples of good practice.
With all necessary materials in place, the process was run with cross-functional teams in 2021 and 2022 across all Novartis franchises covering more than a dozen major disease areas, from leukaemia to cardiovascular disease, dry eye disease to kidney failure, multiple sclerosis to food allergy. Dozens more i4i Insights Discovery™ Insight Sprints are planned for 2023 and beyond. To institutionalize i4i Insights Discovery™ as the Novartis way to become more patient-centric, live “train the trainer” training courses have been delivered to more than 100 associates working in functions including patient engagement, insights and analytics and training design and delivery. The User Guide, all necessary materials and a series of bite-sized “how-to” training videos are made available to associates on the Novartis intranet.
The introduction of the Novartis i4i Insights Discovery™ process has changed how Novartis associates understand patients and care partners whose lives are affected by the diseases and conditions its drugs and therapies are designed to address. Since May 2020, the process has been created, piloted, refined and codified. At the end of the INTEGRATE and INSPIRE workshops, facilitators use anonymized questionnaires to collect feedback on the process. This is aggregated and used to specify changes and updates to the process, which are made on an annual basis.
More than 15, cross-functional teams in every one of the company’s franchises – as well as in Novartis’s early-stage, Global Drug Development division – have now taken part in an Insight Sprint. These teams represent a total of more than 200 senior associates, exposed to and deeply involved in this innovative insight discovery process. Each team has surfaced and articulated genuine patient and care partner insights specific to their disease or condition, insights that address a pressing key business question.
The process has enabled all teams to elevate existing research outputs into a more “profound and useful”, more insightful understanding of the patient reality, allowing them to do more with less. These insights have been used to develop high-impact action plans that are designed to improve patient health outcomes, as well as quality of life for both patients and care partners. It is currently too early to determine the extent to which the new patient engagement programs have achieved these goals quantitatively. We will report on metricated outcomes in future papers.
Participant feedback
At the end of the three INTEGRATE workshops and following the final INSPIRE workshop, facilitators routinely administer confidential questionnaires. The most relevant findings are summarised in Table 1 , below. The answers to the first four questions in the table – which were asked as counts or on Likert-type scales – have all been harmonised to be an odds ratio, with 0.00 representing “not at all likely to say this” and 1.00 “completely likely to say this”. Across the 70 participants who provided complete feedback, 95% believe i4i Insights Discovery™ to be “an effective and efficient way to discover patient insights”, whereas 90% believe that it “has helped the team to develop and articulate genuine patient insights”. A total of 83% said that the insights created during the process had enabled them and their colleagues to develop action plans that they believe will have a genuine impact on patients’ lives, action plans to which 85% are committed at the end of the process. On the Net Promoter Score (NPS) question ( Reichheld, 2003 ), the average response – given on a slider – was 8.61 out of 10.00, just the wrong side of the “Promoter” side of the NPS 8/9 boundary.
Participants were given the opportunity to describe – in free text answers – how they found the process, what it enabled them to achieve and what they thought about the way insights were leveraged to develop high-impact action plans, particularly in patient engagement. A selection of responses follow:
The process enabled people to look at the patient journey through the eyes of the patient, identifying key pain points to solve for. The ideas that came out of the ideation and problem solving were well thought out, multi-stakeholder in nature, and adaptable/scalable.
We had a lot of information before, but had never been able to distill it down to foundational patient insights.
All of our solutions had the patient as the primary focus, and we involved other stakeholders only as a means of improving the treatment experience for patients. In this way, we can empower patients to live their lives to the fullest – addressing our key challenge.
The process really works; it’s a systematic methodology for obtaining patient insights. It puts you in a different frame of mind – provided you set aside enough time for robust discussions and for getting workflows going from the insights that come forward.
The process was created, piloted, refined and codified under successive COVID lockdowns, designed to be every bit as effective and dynamic online as in the room. This mode of delivery will be necessary in the long-term in any case, as three of Novartis’s global hubs are in Switzerland, the USA and India. It is neither time efficient nor cost effective to bring participants from these three time zones together for a series of half-day workshops held over a two-month period. The first dozen Insight Sprints were run entirely online using Microsoft Teams, and, although post-pandemic country teams are starting to run Sprints in person – at least in part – the fact that the process was designed to run virtually as well as in the room makes it more attractive and useable in the long-term.
Given the flux and unpredictability of the past two years, the long-term trend for increased working from home and the ongoing disruption of corporate realignment and reorganization, the intensive, immersive and intimate nature of the process has also evidently had positive impact on strengthening existing teams and building new team relationships. The following two quotes are typical of the feedback, reflecting one side-benefit of the process being its ability to bond participants together via a common purpose:
It’s an incredibly collaborative process, designed for every individual to contribute their unique perspective. It has been so well structured, with each stage leading to focused outcomes, getting us closer to the goal of patient-centric tactics.
A great collaborative experience that can help build team relationships and understanding while exploring patient insights. A unique approach that can be leveraged to digest patient and healthcare practitioner research to help stimulate further ideation on patient solutions.
Training feedback
Of more than 100 senior Novartis associates who have been trained in how to run i4i Insights Discovery™, more than two-thirds completed post-training evaluation questionnaires. Their ratings of the training are summarized in Table 2 . The scores are averages, with each question scored out of five, with a score of four ranked as “Very good” and five as “Excellent”.
Conclusions
Novartis set out in 2020 to develop an innovative, effective, replicable way of understanding the patient reality better to help improve adherence and compliance to drug regimens and so improve patient outcomes. To become ever-more patient centric, the company sought to introduce an evidence-based – yet creative – methodology to enable cross-functional teams in every franchise to surface and articulate genuine patient insights. To meet patient needs better, it sought to bring the voice, experience and perspective of patients to the heart of drug development, design and delivery. The company wanted to square the circle by creating and institutionalizing a fundamentally insightful thinking approach into its resolutely analytical culture. The goal was to enable brand and product teams, disease by disease, to make more of the research outputs they commission by identifying and capitalizing on alignments and synergies between different data sets and reports.
Meaningful insights into patients’ lives can remain hidden in plain sight. Often, we do not need to do more research, patient and care partner segmentation or social media listening. We need to make better use of what we already know, shuffling and reshuffling “old and old to make something new”. At a time of tightening budgets and demands from leadership to demonstrate better impact, this paper has sought to demonstrate how it has been possible to do more with less; to turn existing research outputs into a more profound and useful understanding of what it means to live with specific diseases or conditions from all perspectives.
Although the process was designed in response to a desire from leadership to become increasingly patient-centric, the psychological and cognitive underpinnings of the process in fact mean that it is stakeholder agnostic. i4i Insights Discovery™ has already been used to develop better-informed, evidence-based action plans, rooted in a deeper understanding of patients and care partners. More research is necessary to assess how the methodology performs when looking to better characterize health-care professionals and health-care systems.
There are two additional areas where further research can assess the impact and efficacy of the process. The first requires Novartis to validate formally the patient insights generated by the process with patients. While many of the insights and clusters/themes of insights have been informally validated with various patient advocacy groups, a more rigorous program of testing and validation of insights created would give teams even greater confidence that the process gives them a better understanding of the patient reality. The second requires a comprehensive impact assessment of the new patient engagement strategies developed from the key challenges and resulting insights, understanding whether these insight-rich action plans do achieve reliable and sustainable impact on compliance and adherence and – in the end – enhance patient outcomes and so quality of life.
The Novartis i4i Insights Discovery™ process has already greatly benefitted Novartis. This is why the company is sharing its learnings to enable others so that they can benefit from this innovative approach. It is Novartis’s intention to make i4i Insights Discovery™ a gold standard in insight discovery.
Participant feedback on taking part in i4i Insights Discovery™ Insight Sprints ( n = 70)
1 Participants in the training also said: “I’ll need to refer to the User Guide, presentations, and templates, but the course gave me a good grounding in the whats, the whys, and the hows of i4i”
Source: Authors’ own work
Allan , D. , et al. ( 1999 ), What If? How to Start a Creative Revolution at Work , Capstone , Oxford .
Amabile , T.M. , et al. ( 2017 ), “ Assessing the work environment for creativity ”, Academy of Management Journal , Vol. 39 No. 5 , pp. 1154 - 1184 , doi: 10.5465/256995 .
American Medical Association ( 2020 ), “ 8 Reasons patients don’t take their medications ”, available at: www.ama-assn.org/delivering-care/patient-support-advocacy/8-reasons-patients-dont-take-their-medications
Berinato , S. ( 2019 ), “ Data science and the art of persuasion ”, Harvard Business Review , Vol. 97 No. 1 , pp. 126 - 137 , available at: https://hbr.org/2019/01/data-science-and-the-art-of-persuasion
Catmull , E. ( 2014 ), Creativity, Inc.: an Inspiring Look at How Creativity Can – and Should – Be Harnessed for Business Success by the Founder of Pixar , Bantam Press , London .
Cropley , D.H. ( 2016 ), “ Creativity in engineering ”, in Corazza , G. and Agnoli , S. (Eds), Multidisciplinary Contributions to the Science of Creative Thinking , pp. 155 - 173 . Springer , Singapore , doi: 10.1007/978-981-287-618-8_10 .
De Bono , E. ( 1985 ), Six Thinking Hats: An Essential Approach to Business Management , Penguin , London .
Felder , R.M. and Brent , R. ( 2003 ), “ Learning by doing ”, Chemical Engineering Education , Vol. 37 No. 4 , p. 282 .
Flesch , R. ( 1951 ), The Art of Clear Thinking , Harper and Row , New York, NY .
Guildford , J.P. ( 1959 ), “ Traits of creativity ”, in Anderson , H.H. (Ed.), Creativity and Its Cultivation , Harper , New York, NY , pp. 142 - 161 .
Janis , I.L. ( 1972 ), Victims of Groupthink , Houghton Mifflin , Boston, MA .
Jansen , AE. and Searle , B.J. ( 2021 ), “ Diverse effects of team diversity: a review and framework of surface and deep-level diversity ”, Personnel Review , Vol. 50 No. 9 , pp. 1838 - 1853 , doi: 10.1108/PR-12-2019-0664 .
Joshi , P.P. , et al. ( 1999 ), “ Factors precipitating congestive heart failure–role of patient non-compliance ”, The Journal of the Association of Physicians of India , Vol. 47 No. 3 , pp. 294 - 295 .
Kahneman , D. ( 2011 ), Thinking, Fast and Slow , Penguin , London .
Kelley , T. ( 2001 ), The Art of Innovation: Lessons in Creativity from IDEO, America's Leading Design Firm , Bantam Doubleday Dell , New York, NY .
Knoblich , G. , Stellan , O. , Haider , H. and Rhenius , D. ( 1999 ), “ Constraint relaxation and chunk decomposition in insight problem solving ”, Journal of Experimental Psychology: Learning, Memory, and Cognition , Vol. 25 No. 6 , pp. 1534 - 1555 , doi: 10.1037/0278-7393.25.6.1534 .
Knowles , S.K.Z. ( 2018 ), Narrative by Numbers: How to Tell Powerful and Purposeful Stories with Data , Routledge: Taylor and Francis , Abingdon , available at: www.narrativebynumbers.com
Knowles , S.K.Z. ( 2020 ), How to Be Insightful: Unlocking the Superpower That Drives Innovation , Routledge: Taylor and Francis , Abingdon , available at: https://HowToBeInsightful.com
Knowles , S.K.Z. ( 2022 ), Asking Smarter Questions: How to Be an Agent of Insight , Routledge: Taylor and Francis , Abingdon , available at: https://asksmarterqs.com
Kostev , K. , et al. ( 2014 ), “ Physicians’ influence on breast cancer patient compliance ”, GMS German Medical Science , Vol. 12 No. 3 , doi: 10.3205/000188 .
Kounios , J. and Beeman , M. ( 2015 ), The Eureka Factor: Creative Insights and the Brain , Windmill Books , London .
Laederach-Hoffman , K. and Bunzel , B. ( 2000 ), “ Noncompliance in organ transplant recipients: a literature review ”, General Hospital Psychiatry , Vol. 22 No. 6 , pp. 412 - 424 , doi: 10.1016/S0163-8343(00)00098-0 .
Lowes , J.L. ( 1927 ), The Road to Xanadu: A Study in the Ways of the Imagination , Houghton Mifflin , Boston, MA .
MacGregor , J.N. , Ormerod , T.C. and Chronicle , E.P. ( 2001 ), “ Information processing and insight: a process model of performance on the nine-dot and related problems ”, Journal of Experimental Psychology: Learning, Memory, and Cognition , Vol. 27 No. 1 , pp. 176 - 201 , doi: 10.1037/0278-7393.27.1.176 .
Mayer-Schonberger , V. and Cukier , K. ( 2013 ), Big Data: A Revolution That Will Transform How We Live, Work and Think , John Murray , London .
Mlodinow , L. ( 2018 ), Elastic: Flexible Thinking in a Constantly Changing World , Allen Lane , London .
Moore , E.M. ( 2007 ), “ How to kill creativity – ten easy steps ”, Industry and Higher Education , Vol. 21 No. 5 , pp. 337 - 343 , doi: 10.5367/000000007782311876 .
Morris , L.S. and Schulz , R.M. ( 1992 ), “ Patient compliance – an overview ”, Journal of Clinical Pharmacy and Therapeutics , Vol. 17 No. 5 , pp. 283 - 295 , doi: 10.1111/j.1365-2710.1992.tb01306.x .
Pink , D. ( 2014 ), To Sell is Human , Canongate Books , London .
Pollock , L. ( 2021 ), The Book about Getting Older , Penguin Random House , London .
Reese , H.W. ( 2011 ), “ The learning-by-doing principle ”, Behavioral Development Bulletin , Vol. 17 No. 1 , pp. 1 - 19 , doi: 10.1037/h0100597 .
Reichheld , F. ( 2003 ), “ The one number you need to grow ”, Harvard Business Review , Vol. 81 No. 12 , pp. 46 - 54 .
Roberts , D. ( 2018 ), “ The engagement agenda, multimedia learning and the use of images in higher education lecturing: or, how to end death by PowerPoint ”, Journal of Further and Higher Education , Vol. 42 No. 7 , pp. 969 - 985 , doi: 10.1080/0309877X.2017.1332356 .
Rotberg , B. , et al. ( 2020 ), “ Caring about caregivers: the role of paediatricians in supporting the mental health of parents of children with high caregiving needs ”, Archives of Disease in Childhood , Vol. 105 No. 11 , doi: 10.1136/archdischild-2019-318729 .
Storr , W. ( 2020 ), The Science of Storytelling: Why Stories Make Us Human, and How to Tell Them Better , William Collins , Glasgow .
Wallas , G. ( 1926 ), The Art of Thought , Reprinted 2014 by Solis Press , Tunbridge Wells .
Webb Young , J. ( 1940 ), A Technique for Producing Ideas , Reprinted 2003 by McGraw Hill Education , Columbus, OH .
Wenzel , M. and Henne , N. ( 2014 ), “ Beyond the pill: the move towards value-added services in the pharmaceutical industry ”, Journal of Medical Marketing: Device, Diagnostic and Pharmaceutical Marketing , Vol. 14 Nos 2/3 , pp. 91 - 98 , doi: 10.1177/1745790414556564 .
Zisook , S. and Gammon , E. ( 1981 ), “ Medical noncompliance ”, The International Journal of Psychiatry in Medicine , Vol. 10 No. 4 , pp. 291 - 303 , doi: 10.2190/6WTD-LNY8-FPB1-LV7N .
Corresponding author
About the authors.
Samuel Kenneth Zachary Knowles is the Founder and Chief Data Storyteller of the consultancy Insight Agents. He helps organizations use data better – in the questions they ask to surface the right data; in the insights they articulate using this data; and, in the insight-rich, data-driven, but fundamentally human stories they tell. He’s the author of the Using Data Better trilogy of books with Routledge, comprising Narrative by Numbers (2018), How To Be Insightful (2020) and Asking Smarter Questions (2022). Sam holds a doctorate in psychology, one source of his understanding of human motivation and behavior and his love of telling stories with data. He is a Fellow of both the Royal Society of Arts and the Professional Speaking Association. He chairs the Data Storytelling Council of I-COM, a global association helping companies achieve competitive advantage through smart data marketing. An experienced and sought-after keynote conference speaker, trainer and consultant, Sam is the cofounder and co-host of the Small Data Forum podcast, a sideways look at the uses and abuses of data big and small in business, politics and public life.
Beyza Klein is an accomplished Global Patient Engagement Director with a focus on Insights and Measurements, responsible for the design and implementation of methodologies to drive patient insights. Her remit includes innovation and developing new tools and platforms to ensure sustainability of new approaches. Patient insight-driven decision-making in health care has been the sole focus of her career. From an educational perspective, she is a sociologist with a postgraduate degree from the University of Sydney. She lives in Basel, Switzerland, working out of Novartis’s HQ campus and in partnership with all divisions, countries, regions and patient organizations across all disease areas where Novartis has efforts to reimagine medicine. Being a sociologist, she has conducted research using a wide variety of methodologies and published research in peer reviewed journals. It is Beyza’s passion and purpose to elevate the patient voice, through evidence, to all health-care stakeholders.
Related articles
All feedback is valuable.
Please share your general feedback
Report an issue or find answers to frequently asked questions
Contact Customer Support
Understanding the real-world patient journey and unmet needs of people with hidradenitis suppurativa through social media research
Affiliations.
- 1 Department of Dermatology, Penn State Milton S Hershey Medical Center, Hershey, PA, USA.
- 2 Department of Dermatology, Hospital de Manises, Valencia, Spain.
- 3 European Hidradenitis Suppurativa Foundation e.V., Dessau, Germany.
- 4 University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, USA.
- 5 Novartis Pharma AG, Basel, Switzerland.
- 6 HS Ireland, Hidradenitis Suppurativa Association, County Clare, Ireland.
- 7 Danish Hidradenitis Suppurativa Patients' Association (HSDK), Copenhagen, Denmark.
- 8 Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau, Brandenburg Medical School Theodor Fontane and Faculty of Health Sciences Brandenburg, Dessau, Germany.
- PMID: 37002732
- DOI: 10.1093/bjd/ljad104
- Hidradenitis Suppurativa* / therapy
- Social Media*
Grants and funding
- Novartis Pharma AG
- Good Publication Practice (GPP4)

A Revolutionary Cancer Therapy Made From Patients’ Cells
Car-t cell therapy, one of the first “living drugs” for cancer, reprograms a patient’s immune cells to help fight the disease..

Scientists and doctors have a new cutting-edge tool to treat certain types of advanced B-cell blood cancer that’s completely different from any treatment that’s come before.
Chimeric antigen receptor T (CAR-T) cell therapy takes a patient’s T cells – the white blood cells that protect the body from infection and disease – and reprograms them into a personalized and one-time treatment that can detect and destroy cancer cells.
It’s a major advancement from many conventional cancer therapies that are delivered uniformly to large groups of patients and must be administered over a period of time.
Novartis collaborated with the University of Pennsylvania to commercialize the first of two CAR-T cell therapies currently approved by the U.S. Food and Drug Administration for certain types of advanced B-cell blood cancers.
“We knew that we had a potential therapy that could be effective at treating untreatable cancers,” says Dr. Bruce Levine of the University of Pennsylvania, one of Penn’s cell therapy and biologics manufacturing experts. “We felt the most ethical pathway was to get the therapy developed in the fastest way possible with a company that had global oncology expertise, as well as clinical and regulatory expertise. Our alliance with Novartis successfully developed what would become the first approved CAR-T cell therapy for patients.”
Follow one patient’s journey with CAR-T cell therapy to learn how scientists and healthcare professionals are pioneering new approaches at each step of this innovative, individualized therapy, with the goal of bringing CAR-T to more patients around the world.

The CAR-T Journey Begins
The patient, who had a type of B-cell blood cancer, underwent multiple unsuccessful rounds of chemotherapy and a stem cell transplant. Soon after the transplant, doctors discovered the patient’s cancer had returned and spread to his lungs, becoming Stage 4, or advanced cancer.
“A few nurse practitioners told my wife this was it, that my life expectancy was about three to six months,” the patient says. “We were told to get our affairs in order.”
Yet, the patient’s doctor encouraged him not to give up and told him about CAR-T cell therapy.
“Is it a plan for a potential cure? the patient asked his care team. “Because if it may be a cure, I’m all in!”
CAR-T cell therapy has the potential to halt the progress of the disease. And while long-term follow-up data isn’t yet available, CAR-T cell therapy is a “living drug,” meaning that if the therapy is successful, the patient goes into remission and the cells continue fighting disease, helping the patient stay cancer-free.

CAR-T cell therapy is a “living drug,” meaning that if the therapy is successful, the patient goes into remission and the cells continue fighting disease, helping the patient stay cancer-free. @import url('https://fonts.googleapis.com/css?family=Muli&display=swap');
Creating living drugs from t cells, creating living drugs from t&nonbreakingspace;cells.
After the patient conferred with his care team, he decided to try CAR-T cell therapy. His doctor referred him to a certified treatment center, which had specialized teams trained to collect and infuse cells and care for him during and after the process.
There, the patient’s T cells were collected through a process called leukapheresis. “It’s a low-risk procedure,” says Dr. Richard Maziarz, a Professor of Medicine at Oregon Health and Science University, and a hematologist who specializes in blood cancers. “It’s similar to donating platelets at the Red Cross, where blood gets spun through a centrifuge and then divided into its parts. But in this case, we’re collecting the white cells.”

@import url('https://fonts.googleapis.com/css?family=Muli&display=swap');
How car-t identifies and destroys cancer cells.

Step No. 1 T cells play a central role in fighting disease, yet they need help recognizing cancer cells.
Step no. 2 at a certified facility, blood from the patient is removed and separated to collect white blood cells, including t cells (leukapheresis). those cells are then sent to a manufacturing facility for reprogramming..

Step No. 3 The patient’s T cells are genetically modified to recognize a specific marker on some cells, including cancer cells.

Step No. 4 The reprogrammed cells, now CAR-T cells, are multiplied and given rigorous quality tests before being shipped back to the certified facility.

Step No. 5 The patient receives the modified cells, which begin identifying markers on cancerous and healthy cells, attaching to them and initiating cell death.
Step 1 t cells play a central role in fighting disease, yet they need help recognizing cancer cells. step 2 at a certified facility, blood from the patient is removed and separated to collect white blood cells, including t cells (leukapheresis). those cells are then sent to a manufacturing facility for reprogramming. step 3 the patient’s t cells are genetically modified to recognize a specific marker on some cells, including cancer cells. step 4 the reprogrammed cells, now car-t cells, are multiplied and given rigorous quality tests before being shipped to the certified facility. step 5 the patient receives the modified cells, which begin identifying markers on cancerous and healthy cells, attaching to them and initiating cell death., safeguarding the patient’s cells.
After collection, the patient’s T cells were sent for reprogramming — but not before they were labeled with a unique ID and bar code to ensure the CAR-T cell therapy he would later receive was the one made from his own cells. Novartis has established a meticulous chain-of-identity system in which all stakeholders involved in the process continually verify patient identity by scanning a clearly marked label unique to each patient.
“The manufacturing of CAR-T cells is a highly sophisticated, circular process that starts and ends with the patient, so chain-of-identity is crucial and one of our top priorities,” says Jonathan Smith, Novartis’s global head of strategy and project management, cell and gene manufacturing.
Reprogramming T Cells
When the patient’s T cells arrived at the CAR-T manufacturing facility, teams of scientists used an inactive virus to reprogram them to hunt an antigen found on both cancerous and normal B cells. Next, scientists incubated the CAR-T cells so they could multiply into large quantities that could be infused back into the patient.
Novartis manufacturing sites work diligently to produce CAR-T cell therapy. The company is globally expanding its manufacturing sites to give more patients access to CAR-T. “For many of these patients,” Smith says, “this could be the last line of treatment, so time is critical. The closer we are to patients within their regions of the world, the faster we can provide them with the treatments they need.”

'For many of these patients, this could be the last line of treatment, so time is critical.' @import url('https://fonts.googleapis.com/css?family=Muli&display=swap'); JONATHAN SMITH, NOVARTIS’S GLOBAL HEAD OF STRATEGY AND PROJECT MANAGEMENT, CELL AND GENE MANUFACTURING
Speed and accuracy are key to the cell production process because CAR-T patients need treatment quickly. As a consequence, some patients may receive what’s called bridging chemotherapy while their T cells are being reprogrammed. “The goal is to keep the cancer under control while we wait for the CAR-T cell therapy infusion,” Dr. Maziarz says.
Infusing CAR-T Cells
Before the patient’s reprogrammed cells were delivered to the certified treatment center so they could be infused back into the bloodstream, he received a course of low-dose chemotherapy known as lymphodepleting chemotherapy. “The goal is to suppress a patient’s immune system,” Dr. Maziarz says. “This gives the CAR-T cells room to grow rapidly and activate against the cancer.”
“The infusion procedure was surprisingly quick and easy,” the patient says. After CAR-T infusions, doctors closely monitor all patients for serious side effects.

‘For many of these patients, this could be the last line of treatment, so time is critical.’ @import url('https://fonts.googleapis.com/css?family=Muli&display=swap'); JONATHAN SMITH, NOVARTIS’S GLOBAL HEAD OF STRATEGY AND PROJECT MANAGEMENT, CELL AND GENE MANUFACTURING

Keeping Watch for Side Effects After CAR-T Infusion
Keeping watch for side effects after car‑t infusion.
After the CAR-T infusion, the patient stayed in the hospital for a week to monitor for potential side effects.
While some patients say CAR-T is easier to tolerate than chemotherapy or a bone marrow transplant, the treatment has its own risks, some of which are serious and life-threatening. “We see side effects ranging from mild flu-like symptoms to severe symptoms that require intensive care unit support,” says Colleen Callahan, a nurse practitioner in the Cell Therapy and Transplant Program at Children's Hospital of Philadelphia.
The most common side effects of CAR-T cell therapies are conditions called cytokine release syndrome (CRS) and neurotoxicity, both of which can lead to death in some cases. Both result from an inflammatory response that may happen in the body as CAR-T cells rapidly attack the cancer.
Cytokine release syndrome can cause fever, low blood pressure and other symptoms, while neurotoxicity can cause delirium and seizures. Doctors closely monitor CAR-T patients to quickly diagnose serious side effects and manage them appropriately.
“We’ve learned we can reduce the side effects by monitoring and treating the symptoms early,” says Dr. Eneida R. Nemecek, a professor of pediatrics and medical oncology at Oregon Health and Science University who was involved in early clinical trials for CAR-T. “There are a variety of anti-inflammatory drugs that we can use to reduce side effects without impacting the CAR-T treatment response.”

‘It’s so exciting to see patients and their families when they come back to see us months after treatment.’ @import url('https://fonts.googleapis.com/css?family=Muli&display=swap'); NURSE PRACTITIONER IN THE CELL THERAPY AND TRANSPLANT PROGRAM AT CHILDREN'S HOSPITAL OF PHILADELPHIA
Monitoring, recovery and long-term follow-up.
The patient saw his doctor for a checkup one month after the procedure and again three months later. He continued to check in with doctors every three months so they could measure CAR-T’s effect on his cancer.
“It’s so exciting to see patients and their families when they come back to see us months after treatment,” says Callahan, the nurse. “Many times, they’re going back to school, hanging out with friends and living normal lives.”
Many early responses to CAR-T have been promising, doctors say. “I’ve seen a lot of patients go into a complete remission by Day 30,” Dr. Maziarz says. “I’ve seen others who still have disease at Day 60. But by Day 90 or 120 it starts to disappear.
“The theoretical possibility is that, with this one-time treatment, the CAR-T cells will stay viable and continue reproducing in patients and targeting the cancer over many years.”

The Future of CAR-T and a New Era of Cancer Treatment
Because CAR-T is a personalized, cell-based approach compared to a traditional therapy, scientists continue to study how it can be improved and how its impact can be expanded. They hope to one day use CAR-T to treat blood cancers in earlier stages and even address other types of cancer, including solid tumors.
As with all cancer treatments, not every patient will respond to CAR-T cell therapy, and some who initially respond may relapse over time. That’s why Novartis scientists are pursuing clinical research that will help them better understand cancer relapse and why patients respond or don’t respond to CAR-T. They hope to integrate this research into Novartis therapies and continuously improve them.
Yet scientists are keenly aware that time is working against many cancer patients, who can’t wait months to receive CAR-T. That’s why Novartis is working to enhance and update the CAR-T manufacturing process and expand production sites, allowing more patients to receive potentially life-saving therapies quickly.
“Our scientific teams are dedicated to identifying and driving forward innovative approaches to CAR-T therapy," says Dr. Jay Bradner, the president of the Novartis Institutes for BioMedical Research. "We have learned a great deal about specific drivers of resistance and relapse from our initial trials and are now applying this knowledge to next-generation technologies, targets and combination strategies so we can deliver transformative treatment options for patients."
Illustration and Animation by Raphaëlle Martin

The news and editorial staff of The New York Times had no role in this post’s creation.
Search form

Story : Transforming new product commercialization in pharmaceuticals: how patient journey became the foundation of marketing planning at Novartis
The healthcare environment is going through a period of unprecedented change and complexity due to new regulations and pricing pressures that has created significant limitations on how the pharmaceutical industry can operate in the evolving marketplace. This also means that established processes for commercializing new pharmaceutical products are now increasingly becoming obsolete thereby compelling sales and marketing teams to re-engineer their processes in a changing world -where rules of the business are getting redefined by the day. At Novartis, we were able to address this issue head-on by building deep organizational capabilities for customer journey mapping through an organization wide innovation program whereby the strategic role of patient journey mapping became an enabling framework to transform our product plans and established a completely new approach to understanding the evolving stakeholders in the healthcare journey. Further our team was able to take this innovative approach to the next level by helping internal marketing teams leverage this approach in designing the right organization blueprints for our new launches with recommendations for appropriate team structures, skills, capabilities and roles within the pharmaceutical selling organization to create winning business models for our new product launches.
"To meet today’s demands and lower costs, we must shift away from the traditional “transactional” healthcare approach. I see a future of healthcare systems that is focused on delivering positive patient outcomes. Instead of rewarding stakeholders for simply providing care, value would be placed on products, services or business models that incrementally benefit patients or reduce costs."- Joseph Jimenez, CEO Novartis
Novartis is a world leader in the research, development, manufacturing and marketing of products to protect and improve health and well-being. Our goal is to discover, develop and successfully market innovative products to prevent and cure diseases, to ease suffering and to enhance the quality of life. We also want to provide a shareholder return that reflects outstanding performance and to adequately reward those who invest ideas and work in our company.
In 2010, a key internal challenge that was identified within the General Medicines department of the Pharmaceuticals division was a lack of a consistent approach within product teams in mapping the healthcare journey of the patient in moving from the early stages of the disease through all stages of treatment until final outcome or cure. This was particularly important for the organization because in order to map the rapidly evolving healthcare marketplace and to stay ahead of the competition, product teams needed to obtain a much better grasp of the key interlinkages between different stakeholders as well as the different challenges and bottlenecks that patients faced across priority disease areas so that we could better address real patient needs.
Thus, a lack of systematic approach and methodology led to significant gaps in developing a meaningful and comprehensive commercialization strategy for new products as teams were often missing out on key insights and opportunities which led to sub-optimal marketing strategies in their product plans. Developing comprehensive customer journey maps was a complex endeavor because it involved assimilating customer information from multiple functions where information often existed in silos and need for deeper cross-functional team engagement to gather meaningful insights in the marketplace was often at cross-purposes with low priority given to this exercise within annual product planning cycle. Further, patient journey was seen to be a marketing functional deliverable only with limited participation by other critical organizational functions such as medical or market access functions within the overall planning process.
This issue was further exacerbated by the fact that there was also not a organization-wide common approach to understanding the patient journey across product teams in global, regions and country functions leading to a fragmented customer insights landscape worldwide that prevented effective customer strategy development at all levels.
Our team addressed this topic by developing a roadmap and toolkit for mapping the patient journey- we called it the Unified Patient Journey framework and developed detailed guiding materials and supporting tools to help product teams develop a comprehensive understanding of patients needs along the entire treatment journey.
Together with senior management, development of high quality patient journeys within the product planning cycle was identified as a core strategic capability for which a cross-functional core innovation team was appointed. As the innovation team started engaging the organization through a series of workshops and round-tables on this critical topic, core definitions and business rules around this project emerged and was incorporated within planning guidelines creating a spirit of co-creation and co-ownership across the organization that led to a flood of new innovative ideas on this topic.
For example one of the workshop teams came to the conclusion that given the confusion around the terminology of the patient journey and lack of clarity within teams on how to best leverage the putputs, a clear definition was needed for the organization on what it was. The team agreed that - the patient journey is a systems based approach to understand how an individual becomes a patient and how they move through the healthcare system with mapped interaction points with each stakeholder in the journey.
Further the above workshop team also identiied the outcome of the project in terms of clear benefits for the business and the team agreed that completing this exercise as planned would enable us to uncover the most critical unmet patient needs where we could offer superior value propositions vs competitor products available in the market.
In hindsight, articulating a clear definition of what we were trying to do and defining clearly what the business benefits were helped immensely in aligning the business and moving faster with implementation than we had previously anticipated.
Also for the first time , the coordination team fostered a truly cross-functional approach to mapping the patient journey and each of the functional leaders were fully engaged from the very beginning. For example, to improve the engagement of critical non-marketing functions such as medical affairs and market access, each functional leader was asked to imagine how their function could benefit from the patient journey exercise and were asked to submit functional checklists and questions that got incorporated within the guidance manual for product teams.
Further, learning manuals and quick summary guides together with live workshops were used to embed this new approach within annual marketing plans. This led to a consistent worldwide approach to mapping the patient journey thereby helping the organization develop a common language and vocabulary while maximizing the value of dialogue and teamwork between global functions, regions and countries.
A key outcome of this approach was that teams started envisioning their current and future patient journeys leading to clear benefits that helped product teams create innovative business models around new launches.
Current patient journey- was developed by populating the framework with available market insights with contribution from market research, sales and other functions with direct customer interactions
Future patient journey - was developed through live cross-functional workshops where each team were asked to imagine what the ideal patient journey would look like taking into account the biggest gaps and unmet needs within the current patient journey. Each contribution and idea was colour coded by category and grouped together on a flip chart (see photo) and thereafter screened through a prioritization exercise.
The gap between the current and future patient journey would then lead to identifying critical hypotheses that teams would brainstorm together to develop future business models. Some questions that emerged were -
-What healthcare problems are our customers trying to solve within the patient journey today and what are their biggest frustrations with the pharmaceutical industry?
-Are solutions to our customers needs available in-house or do we need to find the right partners to create the right beyond the pill solutions for our customers ?
-What skills, capabilities and roles do we need in order to be successful with the right sales team and ideal business model for realizing the vision of the future patient journey ?
-What services and programs do we need to complement our selling organization and complete the business model to commercialize successfully ?
One early challenge the team came up with was that certain groups within the organization wanted to champion their own patient journey approach and in particular 2 of these groups wanted to drive their own preferred framework. To address this conflict, the coordinating team invited members of both these groups to cross-functional workshops and also addressed their pet issues by reframing the key needs to a step earlier than the framework in terms of golden rules that all great patient journeys should follow. Having agreed to the common definition, the teams came up with 8 golden rules that should be part of all completed patient journeys. For example one of the rules was that the patient journey framework must truly be a end-to end mapping exercise and must illustrate the patient pathway from pre-diagnosis to diagnosis through treatment and final outcome. Another rule that was agreed by the team was that the framework must be able to capture all stakeholders in the healthcare journey and not just physicians alone. By spending time upfront in workshops and coming up with these golden rules helped the project team ultimately move faster as those existing fameworks that did not embed all the golden rules were quickly rejected and the team was able to come up with a final patient journey framework that incorporated all the golden rules.
Another observation post implementation of this project was that although teams worked cross-functionally in completing their patient journeys, there was limited knowledge sharing of patient journeys across different product teams leading to incomplete understanding of common issues and knowledge gaps. To address this issue, the coordinating team organized a full day workshop where all completed patient journeys were displayed side by side in the conference venue and each team was asked to comment on key insights from their own journey. A major benefit from this exercise was that teams went away with a much better understanding of common issues and challenges as well as formed joint working groups to address common issues together. For example one such group was formed when the hypertension and diabetes product teams agreed that due to high overlap of these diseases within the population as a co-morbid sub group, it made sense to look at the journey of those patients who were afflicted with both conditions, thus creating a common sense of purpose within these teams to address some of the common levers that emerged from the patient journey exercise.
Having established the new approach, 2 years on, a number of key benefits heve emerged-
-Product cross-functional teams across global, regions and countries can now engage in common dialogue and discussion around key insights around the patient journey as well as benefit from a much more constructive engagement throughout the planning process
-Where there are significant differences in country market healthcare landscape, teams often come up with different sets of opportunities within their local patient journeys - for example in certain countries healthcare restrictions prevent them from engaging with patients directly through patient programs whereas in other countries teams can develop such programs with minimal regulatory challenges.In these instances, a common framework fosters a strategic dialogue on differential opportunities in markets based on local business rules and unmet needs
-As strategy is about fundamentally articulating where we will focus and also where we will not focus, the completed patient journey has become a useful strategic communication tool for product teams to be able to explain their strategy of focussing on patient segments with the highest unmet needs while de-prioritizing lower priority segments across the journey.
-Rather than product teams stopping at completing their current patient journeys within their planning process, for the first time, teams were able to develop a vision for an aspirational patient journey-in other words, what would the ideal future look like in a world where our products and services come together with the right team with a clear vision for commercial success.
-Creation of the future world through the above step became the template for launch planning by unleashing new innovation within product teams helping them create new organization blueprints for launches with people and services that would address patient and customer needs better than the competition.
-Once the patient journey framework was in place within the planning cycle and it became routine for teams to plan in this manner, this unleashed a further wave of innovation that emerged within countries with minimal global guidance. For example a couple of country teams developed a innovative patient journey app on an ipad that became a customer engagement tool to map hospital patient journeys and supported identifying critical unmet needs within the hospital so teams could come up with the right solutions to address needs of their customers within those hospitals.
-Customer journey mapping should be an organization wide exercise rather than limited to marketing responsibility only in order to unleash innovation at all levels within the organization. Sales function -in particular for already launched products- are critical stakeholders who need to be at the table during this exercise and can bring tremendous value in representing customer insights gained through their day to day interactions.
-Understanding and mapping the current customer journey itself has limited value. Organizational teams should invest time, resources and energy to develop future customer journeys imagining new future worlds and touchpoints between their products, teams, services and consumers that they seek to create and nurture. In times of significant uncertainty within the external environment which can sometimes cause paralysis of decision making particularly within product teams, such an exercise can become a powerful catalyst in mobilizing the organization at all levels -particularly the middle and lower ranks who may feel dis-enfranchised due to their limited input and impact on customer strategy development.
- Aligning global, regional and country organizations through a common customer mapping framework and processes can unleash innovation by fostering greater dialogue and partnership in co-creation of new business models while creating a sense of one team and significantly minimizing territorial boundaries. Further teams can benefit immensely by incorporating customer journey mapping within the annual planning exercise as it allows them to continuously hit the refresh button and revisit their assumptions and business models in light of an ever changing environment and evolving competitive landscape.
-Beyond its role in helping create new business models within a changing environment, the next wave of innovation will emerge from co-created customer journeys where customers will play an active role in creating and utilizing these customer journey maps sometimes even championing the creation of such maps within their own offices and hospitals. This new wave of innovation will also force traditionally product centric industries such as pharmaceuticals to become more value and solution centered enabling them to think of new solutions and business models to partner with external providers to create appropriate product+solution value propositions that best address the most pressing challenges within healthcare in the 21st century and deliver better patient outcomes further enhancing overall benefits to society.
-Global Commercialization and GPS&C team members
You need to register in order to submit a comment.
- Join the MIX now

This is a nice initiative. Specially when it comes to healthcare, the terms has to be specific. We cannot compromise with a patient's life . So the more will be the facilities, more will be its advantages.
http://feelday.com
- Log in to post comments

Debraj so pleased that Novartis has enbraced the need for personalised approaches/solutions to addressing clinical and non-clinical pressing needs of stakeholders/customers. For far too long Pharma have attempted to push their own short term customer focused strategies that are adding no 'value' for anyone in the healthcare value chain. Your approach to supply agreed needs based solutions wraped around your disease and therapy areas is welcome news. This level of transparency and willingness to provide services and offerings will resonate well with clinical and budgetary stakeholders in many health economies. Economies are facing enormous challenge over the next few years with crippling non-communicable disease rates, unsustainable healthcare infastructures, low resources and lets face it no more money. Have you thought about how mobile health will support the development and delivery of some of your solutions/offerings? Certainly this technology when deployed, for example with remote patient monitoring, disease management and wellness and education, offers the potential for improved clinical management, behaviour modification, education and wellness, and all done in a more affordable way.

Thanks for your comments Michael. Fully agree that mobile health will radically transform healthcare delivery so we should definitely integrate it where possible within our programs. A good place to start would be to go and visit customers as we are developing our patient journey and see what they are doing in this space. Some of the larger hospitals and healthcare systems are more advanced than pharma in embracing these technologies in multiple areas that you have mentioned so there is a great opportunity for us to learn from them.
You are very welcome Debraj, maybe Novartis and my organisation, The GSMA, might like to work together to engage with your customers to undertake a review of the opportunity within your programs and develop some hypothesis/concepts for mHealth services?
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 9
- Patient journey and timeliness of care for patients with breast cancer in Africa: a scoping review protocol
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-4487-5015 Anteneh Ayelign Kibret 1 , 2 ,
- http://orcid.org/0000-0001-8394-6077 Heng Jiang 1 , 3 ,
- Haifeng Yang 4 ,
- http://orcid.org/0000-0003-0877-0424 Chaojie Liu 1
- 1 School of Psychology and Public Health , La Trobe University , Melbourne , Victoria , Australia
- 2 Department of Human Anatomy , University of Gondar , Gondar , Ethiopia
- 3 The University of Melbourne School of Population and Global Health , Melbourne , Victoria , Australia
- 4 Hubei University of Chinese Medicine , Wuhan , Hubei , China
- Correspondence to Anteneh Ayelign Kibret; antesha04{at}gmail.com
Introduction Cancer is the leading cause of death worldwide, with breast cancer being one of the most commonly diagnosed types. Low-income and middle-income countries account for nearly half of all breast cancer cases and related fatalities. In Africa, mortality rates are higher and survival rates are lower compared with developed countries. Timeliness of care is a critical aspect of healthcare, but various studies and healthcare systems use different criteria and methods to measure it. Assessing the breast cancer care pathway and understanding the determinants of delayed care are essential for effective interventions. Therefore, this scoping review aims to evaluate the methods used to measure the timeliness of breast cancer care, identify specific points in the care pathway where delays are most frequently reported, and examine the factors affecting the timeliness of breast cancer care in Africa.
Methods and analysis We will conduct this scoping review using the Arksey and O’Malley framework endorsed by the Joanna Briggs Institute. A scoping review of articles written in English concerning the timeliness of breast cancer care in the African context will be undertaken. Six electronic databases (MEDLINE, EMBASE, CINAHL, SCOPUS, WEB Of SCIENCE and PsycINFO) will be searched to identify published literature on timeliness of breast care in Africa. Two reviewers will independently screen the articles at both the abstract and full-text stages, guided by predetermined inclusion and exclusion criteria. The full texts of identified studies will be further examined and charted using a data extraction form guided by the Model of Pathways to Treatment framework. Publications describing the time to diagnosis and its associated factors in the contexts of breast cancer will be considered for inclusion, with no restrictions based on date of publication. Studies that are published in languages other than English and that do not focus on the timeliness of care or time-related aspects within the care pathway will be excluded. Evidence will be narratively synthesised and analysed.
Ethics and dissemination Ethical approval is not needed as this scoping review does not involve collecting data from human participants. The results produced from this review will be submitted to a scientific peer-reviewed journal for publication and will be presented at scientific meetings.
- Breast tumours
- PUBLIC HEALTH
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2023-081256
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Strengths and limitations of this study
The scoping review will capture the variations in timeliness measurement along the patient care pathway for individuals with breast cancer in African countries.
The review will vividly illustrate the patient journey through metro-mapping techniques.
The review will only include studies published in English, potentially missing out on valuable research reported in other languages.
Introduction
Breast cancer represents a significant global health challenge, as it is the most commonly diagnosed cancer. According to the latest global cancer burden statistics, in 2022, approximately 2.3 million women were diagnosed with breast cancer globally. 1 Breast cancer affects women in every country and can occur at any age after puberty, with incidence rates increasing as women get older. 2 In low-income and middle-income countries (LMICs) and low-income countries (LICs), the burden is particularly severe, with 552 623 and 71 828 breast cancer cases occurring in these regions, respectively. 3 Furthermore, breast cancer is the predominant cancer type among African women, and its prevalence has been steadily increasing over the past three decades. 4 According to the 2022 Global Burden of Cancer Study (GLOBOCAN) data, 198 342 breast cancer cases were reported in Africa, with 133 520 of those cases occurring in sub-Saharan Africa. 3 5
Breast cancer is the leading cause of cancer-related deaths among women worldwide. 6 In 2022, it resulted in approximately 665 683 deaths globally, with around two-thirds of these fatalities occurring in less developed countries. 3 As reported by GLOBOCAN in 2022, breast cancer resulted in 244 361 deaths in LMICs and 38 234 deaths in LICs. Among the LMICs, India, Indonesia and Nigeria had the highest breast cancer mortality rates. India reported approximately 98 337 deaths, Indonesia had about 22 598 deaths, and Nigeria reported 16 332 deaths from breast cancer. 3 These figures reflect the challenges in healthcare infrastructure and access to treatment. In Africa and sub-Saharan Africa, 91 173 and 68 036 breast cancer-related deaths occurred, respectively. 3 5 Mortality rates are higher and survival rates are lower in Africa compared with developed countries. This disparity can be attributed to a lack of adequate diagnostic and treatment facilities, leading to delays in diagnosis and treatment, and suboptimal cancer care. Over 70% of breast cancer cases in Africa are diagnosed at an advanced stage. 7 8 In contrast, developed countries have effectively implemented early detection strategies, facilitated timely access to diagnosis and ensured the widespread availability of effective treatments. 9
Timely diagnosis plays a crucial role in improving patients' journey from cancer symptom awareness to treatment and enhancing survival rates. 10 Unfortunately, the majority of breast cancers are diagnosed at a late stage, when treatments are less effective and more costly. 11 Advanced disease at the time of diagnosis not only leads to reduced survival rates for patients but also often requires more complex and expensive treatments that may not be easily accessible to everyone. This situation limits treatment options, potentially reducing effectiveness and impacting quality of life, affecting daily activities and emotional well-being. This situation also further strains healthcare systems already facing significant challenges. 12 As a result, breast cancer significantly impacts women in the prime of their lives in Africa, leading to pronounced familial, societal and economic consequences. 13
Regular health screenings, awareness of symptoms and prompt medical attention can help catch diseases in their early stages when treatments are often more effective. Early diagnosis and access to effective treatment play crucial roles in determining outcomes for patients with cancer. 9 They provide several advantages, including higher survival rates, greater treatment success rates, enhanced quality of life and decreased financial burdens. 14
The care journey of patients with breast cancer is influenced by numerous factors. Delays at different stages, from screening and symptom recognition to starting treatment in Africa, are often linked to a mix of patient-related factors such as low educational attainment, limited awareness of breast cancer, use of complementary medicine, financial constraints, 15 and health system-related factors like distance to the nearest healthcare centre, choice of initial healthcare provider, the number of providers consulted before diagnosis, inadequate health insurance coverage, and false-negative diagnostic tests. 16
Timely access to healthcare services has become a priority in public health policies. 17 18 The time interval not only is an indicator of the accessibility of healthcare but also aids in identifying inequalities of care in patient management. 19 20 Due to the absence of appropriate benchmarking to measure timeliness, different studies and healthcare systems may use different criteria and methods. This review will identify the various ways in which timeliness is assessed in African studies and the specific point of the care pathway where delays are most prevalent. Besides, by examining the factors associated with timeliness of care, healthcare providers and policymakers can develop targeted interventions and strategies to address specific issues contributing to delays in breast cancer care.
Moreover, we have reviewed systematic 21 22 and scoping reviews 23 that have been done in Africa. We found that there is a scarcity of information regarding how individual studies measure the timeliness of breast cancer care, and exploration of the entire care pathway for breast cancer in Africa. This review will address the gap in the literature by providing a more holistic view of the care paths of patients with breast cancer in Africa.
Methods and analysis
This protocol follows the methodological framework described in the Joanna Briggs Institute Manual for Evidence Synthesis on scoping review protocol, 24 which was based on Arksey and O’Malley’s methodological framework, 25 Levac et al ’s recommendations for applying this framework, and Peters et al ’s enhancements of the framework. 26 27 The proposed scoping review will be reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist. As recommended by Arksey and O’Malley, 25 the review process will consist of the following: identifying the research question, retrieving relevant studies, selection of studies, charting data, and collating, summarising and reporting evidence.
Stage 1: identifying the research question
This scoping review is planning to answer the following main questions:
How is timeliness of breast cancer care measured in the existing literature in Africa?
What factors of timeliness of breast cancer care have been studied in the existing literature in Africa?
At which specific interval(s) in the breast cancer care pathway are delays most commonly reported?
Stage 2: identifying relevant studies
This scoping review will endeavour to address the research questions comprehensively.
Inclusion criteria
The scoping review will include both peer-reviewed publications including reviews (systematic review or scoping review) and grey literature (grey literature can include academic papers, including theses and dissertations, research and committee reports, government reports, conference papers, and ongoing research).
The population of this scoping review will be adult women patients with breast cancer, diagnosed by clinicians irrespective of histological type and disease stage. The participants of included studies will: 1 be women, 2 be 18 years of age or older, 3 have a confirmed pathological diagnosis of breast cancer, 4 have reported time interval in any time point of breast cancer care.
We want to know when and where patients go to seek cancer care through the healthcare system, and why. Additionally, we want to understand how timeliness in seeking and receiving breast cancer care is measured in the existing literature and to identify specific points in the care pathway where delays are most frequently reported. Finally, we will examine the factors influencing the patient journey in breast cancer care in Africa.
Studies and reports conducted in Africa.
Publication type and status
In order to avoid publication bias, this scoping review will encompass all pertinent research, irrespective of its publication format (such as editorials, book chapters, reports, original articles, theses, conference abstracts), study design, and its publication status (whether it is published or accepted for publication, as well as grey literature). Additionally, prior reviews addressing related subjects will be incorporated, and the reference list will be screened to identify pertinent articles.
Given the constraints of time, available resources and translation capabilities, this review will exclusively consider studies that have been published in English.
The review will encompass all articles published in the field of timeliness and breast cancer care up to the date of the final search.
Exclusion criteria
The review will exclude studies that are published in languages other than English, articles that do not focus on the timeliness of care or time-related aspects within the care pathway, and those published solely as abstracts. Furthermore, studies focusing solely on men will also be excluded from the review.
An academic librarian was consulted on developing the search strategy. The search terms will be based on key terms relevant to ‘breast cancer’, ‘timeliness’ and ‘Africa’ ( table 1 ).
- View inline
Identified keywords and index terms to construct search strategy for the scoping review
An exhaustive search for potentially eligible articles will be conducted in the following databases, MEDLINE, EMBASE, CINAHL, SCOPUS, WEB Of SCIENCE and PsycINFO ( table 2 ). The Google search engine will be used to search for editorials, reports from governments, international agencies and professional associations (WHO, African Cancer Organisation) and grey literature.
Search strategy for different databases
In addition, hand search will be performed for reference lists of the included literature and for any recent publications that are accepted and available online early.
Managing references
The search results will be imported into EndNote (V.X9), a reference management programme that facilitates the storage, display and organisation of records from each database. EndNote will also be employed to generate the reference list for the review. Subsequently, references will be imported into Covidence, a web-based software platform developed by the Cochrane Collaboration. Covidence will aid in documenting the review process, including screening for eligible articles and conducting full-text reviews.
Stage 3: study selection
Covidence will be used to support the review and selection of articles for data extraction. Results from the searches will be uploaded and duplicates removed. The study selection process will consist of a two-step procedure, independently conducted by two reviewers (AAK and HY). Initially, based on the predefined inclusion criteria, both reviewers will screen study titles. In the subsequent step, the reviewers will screen the abstracts of the studies that meet the initial criteria, followed by a review of the full texts. Any discrepancies arising between the two reviewers will be resolved through consensus, with the involvement of a third reviewer (CL) if necessary. A PRISMA-ScR flow diagram will be presented to offer a comprehensive visual representation of the studies that were included and excluded during the process of study selection.
Stage 4: charting the data
The data extraction from each eligible article will be carried out using a specifically developed standardised data extraction form. The data extraction form will be informed by the Model of Pathways to Treatment to map the identified evidence on the timeliness, and time intervals and associated factors of breast cancer. 28
Data will be extracted by the lead author (AAK). The information to be extracted from the included articles will encompass the author’s name, year of publication, country of origin, study design, research setting, study population, sample size, the various time intervals within each stage of the breast cancer care pathway, metrics to measure time interval (delay), and determinants of timeliness ( table 3 ). To ensure uniformity and reliability in the data extraction process and to confirm that all relevant information is captured, two reviewers (AAK and CL) will independently pilot test the extraction template using a subset of the included studies. Following this pilot test, any necessary revisions to the template will be made accordingly.
Data charting form
During the formal data extraction phase, one reviewer (AAK) will carry out the data extraction in accordance with the objectives outlined in this scoping review, and a second reviewer (HJ) will subsequently verify the accuracy of the extracted data.
Stage 5: collating, summarising and reporting the results
Data synthesis will involve the creation of narrative summaries derived from the extracted information and NVivo software will be employed. The findings will be visually represented using tables, charts or graphs as appropriate.
We will employ frequency distributions and descriptive statistics to present data related to the year of publication, country of origin, study design, research setting, study population, sample size, the metrics used by each study to measure time intervals within the breast cancer care pathway.
Determinants linked to the duration of various intervals in the breast cancer care-seeking path will be classified in accordance with the Model of Pathways to Treatment framework and the Anderson’s Health Services framework. The Model of Pathways to Treatment framework specifies the essential events, processes and time intervals that may occur in the period prior to diagnosis and the start of medical treatment and identifies the factors that may influence each interval. The model identifies five key events in the pathway to care: detection of bodily changes, perceived reasons to discuss symptoms with a healthcare provider, first consultation with a healthcare provider, diagnosis and start of treatment. Additionally, it highlights four important intervals between these events: the appraisal, help-seeking, diagnostic and treatment intervals ( figure 1 ). 28 Andersen’s model classifies determinants of healthcare into individual and contextual characteristics and the characteristics are further grouped as predisposing factors (including demographic, health belief and social structure aspects), enabling factors (encompassing family characteristics like income and insurance coverage, community characteristics such as resource availability and proximity to care, and regional factors within the country) and need factors (comprising individual experiences of symptoms and health status). 29 The two frameworks will be combined to link facilitators and barriers of cancer care to key events and intervals ( figure 1 ). From this we will describe what factors have been studied and what are missing.
- Download figure
- Open in new tab
- Download powerpoint
Model of Pathways to Treatment and Andersen’s model of health service utilisation. HCP, healthcare provider.
We will identify the phase of the care pathway where delays are most frequently reported. The breast cancer care trajectories in Africa will be depicted by using the metro-mapping method, 30 recognising that patients with breast cancer often follow various care paths. With the aid of metro-mapping, we will clearly depict the distinct care paths of patients with breast cancer, using different colours to connect and represent their care journeys.
Patient and public involvement
Ethics and dissemination.
Ethical approval is not needed as this scoping review does not involve collecting data from human participants. The results produced from this review will be submitted to a scientific peer-reviewed journal for publication and will be presented at scientific meetings.
Breast cancer continues to be a major public health challenge, especially in LMICs like those in Africa. 31 Early detection and treatment of breast cancer significantly reduce morbidity and mortality rates, greatly improving the chances of survival. 32 33 A delay in the path of breast cancer care can lead to the cancer progressing to a more advanced stage, complicating treatment and potentially diminishing survival rates. 34 Furthermore, such delays can impose a heavier financial burden on both patients and healthcare systems. Advanced breast cancer can also result in intensified symptoms, increased pain and physical limitations, significantly impairing a patient’s overall quality of life. 35 In LMICs, a substantial number of women with breast cancer either initially present with or are later diagnosed with advanced-stage disease (locally advanced or metastatic). 33 African countries are yet to establish universal coverage of a breast cancer screening programme, and they face significant accessibility and affordability barriers. 36 Furthermore, continuity and coordination of care remain a significant challenge in the region. For individuals seeking medical attention, numerous challenges exist. Many in Africa lack awareness of breast cancer, its risk factors and the critical importance of early detection, leading to delayed care-seeking. Cultural and societal stigma, financial barriers, geographical distances to healthcare facilities, and low health literacy further hinder patients' understanding of the significance of early detection and timely treatment. 37
Conversely, care providers in the region confront their own set of challenges. Limited resources, encompassing healthcare facilities, trained personnel and diagnostic equipment, can obstruct their capacity to deliver timely and comprehensive care. A shortage of specialised cancer care centres is prevalent in many African countries, making it challenging for patients to access advanced treatments and expert care timely. 38 39
This scoping review has implications both at the system and care levels. At the system level, the review can provide valuable insights for healthcare policymakers regarding the current state of timeliness in various paths in breast cancer care in Africa. This information can serve as a foundation for the development of targeted policies and strategic plans aimed at enhancing the healthcare system’s capacity to deliver timely care. Besides, it can help to pinpoint where investments in healthcare infrastructure, personnel training and equipment are most urgently required to minimise delays.
In terms of care implications, the review can play an important role in informing the development or refinement of clinical guidelines for breast cancer care in Africa. This ensures that these guidelines place a significant emphasis on the crucial aspect of timeliness in screening, diagnosis and treatment, thereby promoting more effective and timely care for patients with breast cancer across the continent.
Most studies on patient care paths are either conducted in developed countries or led by researchers from developed countries. This can lead to value or context bias, even though developing countries bear most of the cancer burden. This bias may arise because healthcare systems, patient experiences and resource availability in developed countries can differ significantly from those in developing nations. As a result, findings from studies conducted primarily in developed countries may not fully reflect the unique challenges and contextual factors faced by patients and healthcare providers in resource-constrained settings. Therefore, this review will provide valuable information about the breast cancer path in the African context and highlight future research direction.
Generally, the aim of this proposed scoping review is to combine information from both published peer-reviewed studies and grey literature regarding the metrics used to measure timeliness, pinpoint the specific time point along the care pathway where delays are more reported, and indicate the factors influencing the timeliness of breast cancer care in Africa.
This review has certain limitations. First, it will not incorporate a quality assessment or grading of evidence. Second, the review will exclusively consider studies published in English, potentially excluding relevant literature in other languages. By excluding non-English studies, the review may overlook valuable research conducted in other languages.
Ethics statements
Patient consent for publication.
Not applicable.
- Siegel RL , et al
- Ferlay JEM ,
- Zhou L , et al
- Joko-Fru WY ,
- Bukirwa P , et al
- Wilkinson L ,
- Jedy-Agba E ,
- McCormack V ,
- Adebamowo C , et al
- Rutherford MJ ,
- Bardot A , et al
- Moodley J ,
- Cairncross L ,
- Naiker T , et al
- Chao A , et al
- de Sanjose S ,
- Mutebi M , et al
- McKenzie F ,
- Dos-Santos-Silva I
- Bhushan A ,
- Gonsalves A ,
- Tegegne TK ,
- Chojenta C ,
- Loxton D , et al
- Hutajulu SH ,
- Prabandari YS ,
- Bintoro BS , et al
- Dvaladze A ,
- Rositch AF , et al
- Martins T ,
- Hamilton W ,
- Ukoumunne OC
- Lathlean J ,
- Wiafe S , et al
- Adeloye D ,
- Sowunmi OY ,
- Jacobs W , et al
- Ansu-Mensah M , et al
- Peters MDJ ,
- Tricco AC , et al
- Colquhoun H ,
- Godfrey CM ,
- Khalil H , et al
- Walter FM ,
- Webster A , et al
- Alkhawaldeh A ,
- ALBashtawy M ,
- Rayan A , et al
- Stiggelbout A ,
- Griffioen I ,
- Brands J , et al
- Manson EN ,
- Richardson LC ,
- Royalty J ,
- Howe W , et al
- Ginsburg O ,
- Brooks A , et al
- Rivera-Franco MM ,
- Leon-Rodriguez E
- Monteiro MN ,
- Queiroz JF , et al
- Martei YM ,
- Vanderpuye V
- Magwesela FM ,
- Msemakweli DO ,
- Ssentongo P ,
- Agbese E , et al
- Akuoko CP ,
- Sarpong T , et al
Contributors AAK generated the idea, developed the research questions and study methods, conceptualised the review approach and developed the manuscript. CL, HJ and HY contributed to developing the research questions, the review and editing of the manuscript. AAK is the guarantor.
Funding This review protocol is funded by a La Trobe University Research Graduate Scholarship (LTURGS).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Reimagining medicine through data-led transformation
Novartis uses a multi-cloud data analytics platform to optimize operations and accelerate innovation.
5-MINUTE READ
Call for change
For pharmaceutical companies, data is in many ways the lifeblood of the industry. With New Science developments—such as genomics, molecular profiling, biomarkers and patient monitoring devices—more data sets are being generated than ever before. Additionally, new supply chain security, patient services and marketing capabilities are creating a treasure trove of operational, patient and healthcare practitioner data. All these advances will produce new data streams exponentially larger than what companies are currently dealing with. As they do so, the data’s value will exponentially grow. The data-thirsty personalized medicine market alone was valued at $493.1 billion in 2021 and is expected to grow at a 6.2% compound annual growth rate from 2021 to 2028.1
Elizabeth Theophille, Chief Technology Transformation Officer at Novartis and Dr. Petra Jantzer, Senior Managing Director, Global Accenture Partner for Novartis talk about Novartis’ Digital Transformation journey and how their Gartner award-winning, multi-cloud platform supports the big data and analytics strategy employed to ultimately Reimagine Medicine.
Novartis, an industry pioneer, has embarked on an ambitious transformation to become the leading medicines company powered by data science and digital technologies. It knows data are only as good as the tools used to analyze and exploit them. How could it make data from all the nooks and crannies of the business work to revolutionize operations, drug development and commercialization? How could it be ready for the data-rich demands of personalization and advances in new therapies like cell and gene therapies?
Accomplishing these goals was difficult with the company’s legacy IT system, data fragmentation and information silos. Novartis required the flexibility and scalability of cloud technology to consolidate data and support dynamic, future-ready technology that help improves collaboration, insights and innovation. An end-to-end data and analytics solution would offer a broader and deeper view of activities to make business and clinical decisions. The insights it yields would help reimagine medical innovation to get patients life-changing therapies, fast and at a lower cost.
1 Grand View Research, “Personalized Medicine Market Size, Share & Trends Analysis Report By Product (Personalized Medical Care, Personalized Nutrition & Wellness, DTC Diagnostics, Telemedicine, Complementary Medicine), And Segment Forecasts, 2021 – 2028,” May 2021, Grand View Research website , accessed October 4, 2021.
When tech meets human ingenuity
From tech giants to startups and academia, Novartis collaborated with a variety of entities including Accenture on a project with the potential to transform all aspects of the business. We first helped map the stakeholder business value for this journey then created the data and analytics operating model, governance, road map, architecture, centralized data catalogue and platform for a holistic solution that harnesses new technologies like artificial intelligence (AI) and machine learning (ML).
Working with Amazon Web Services (AWS) as the primary cloud provider, Novartis also engaged Microsoft Azure to create a multi-cloud platform, complying with the best clinical and pharmaceutical manufacturing practices and offering capabilities across functions. The powerful analytics capabilities enable Novartis to crunch large (and growing) data sets. At launch, approximately 35% of global company data was on the new platform, with the remaining data planned for migration.
Teams can also develop use cases for new analytics-related projects to explore and scale across the business. This helps teams experiment with potential analytics use cases to solve business challenges and profit from opportunities. In some cases, we help deliver the use case programs.
The platform ingests, unifies and refines more than 9TB of internal and external data from over 80 sources in Development, Commercial, Manufacturing and Quality, and Corporate Business Services at a rate 20% faster than legacy systems. The different types of data are put into a standardized format, which can then be used by teams from across the company to simplify reporting.
Novartis’ advanced analytics platform ingests, unifies and refines:
terabytes is the amount of internal and external data the platform ingests, unifies and refines.
sources in Development, Commercial, Manufacturing and Quality, and Corporate Business Services.
faster than legacy systems.

A valuable difference
Novartis is reinventing its business to drive faster decision-making and bold innovation. Teams now have a smorgasbord of analytics tools, supported by AI and ML, to simplify reporting, augment existing programs with data insights, or create new products and services.
“Data democratization” makes insights accessible to relevant users, efficiently balancing ethical, security and regulatory requirements without creating data bottlenecks. Easily interpretable data enable Novartis’ global workforce, partners and researchers to maximize collaboration, ingenuity and productivity. Novartis’ people and research partners use the new platform to cross-pollinate ideas and develop a library of innovative analytics use cases and data models to be applied across the business.
Previously, it took about two weeks to set up a new use case; now it can be done within one day. The use case development time has also accelerated from 10 days to three days. More than 200 use cases are in the pipeline and 36 are under development. Eleven use cases have been rolled out, including DESIRE, a tool for monitoring clinical trial site risk and performance. The benefits go beyond R&D to encompass all aspects of the business. A patient services use case, for instance, is helping Novartis mine call center feedback to improve marketing reach and campaigns.
Novartis has sparked a digital revolution within its business to support data-driven decision making, predict future trends, optimize operations and spur growth. As data from new sciences and medical technologies grow, Novartis has powerful tools to accelerate drug launches—and improve patient outcomes.
Use cases for new analytics-related projects that Novartis can explore and scale to solve business challenges and exploit opportunities:
from across Novartis in pipeline
up and running
days to development
day to set up
This solution has drastically transformed our business.
Loic Giraud / CoE Lead Business Analytics – Novartis

IMAGES
COMMENTS
Unbranded disease infographic on the TSC patient journey. Tuberous sclerosis complex (TSC) is a genetic disorder that may cause non-cancerous tumors to form in vital organs and can affect many parts of the body, including the brain, kidneys, heart, lungs and skin, with some patients exhibiting seizures, autism, cognitive delays or behavior issues.
Hypothetical Patient Case Study INITIAL PRESENTATION Examination PCP and surgical oncologist Surgical oncologist and Pathologist BIOPSY Ultrasound guided core needle biopsy 1 2 Pertinent results Histology Type Invasive ductal carcinoma Grade 3 Size 3.7 cm in greatest dimension Receptor status ER 80% PR 55% HER2 IHC 1+ Not an actual patient ...
At Novartis, we are exploring how to address unmet needs in prostate cancer by combining science with empathy and partnering with organizations that understand this community. Learning more about the disease and aspects of the journey - including PSMA - can help people diagnosed with prostate cancer make informed decisions together with their loved ones and health care teams about their ...
KYMRIAH is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse. Important Safety Information for KYMRIAH® (tisagenlecleucel) Cytokine release syndrome (CRS ...
Novartis reserves the right to rescind, revoke, or amend this program without notice. If you have already been prescribed LUTATHERA, sign up for Novartis Patient Support. Call 1-844-638-7222, Monday - Friday, 8:00 AM to 8:00 PM ET, excluding holidays. Ask your health care provider (HCP) to help you sign up for assistance, like the Co-Pay Plus ...
The estimand framework was applied to design a trial for a chimeric antigen receptor T-cell therapy. The treatment effect of interest was carefully defined considering the range of patient journeys expected for this particular indication and treatment. The trial design was developed accordingly to assess that treatment effect.
Based on previous literature on patient journey mapping 16,27 and the diagnostic journey in axial spondyloarthritis 2,21,22,26, the initial approach to interview conduction and data coding was a ...
chain and patient journey 7 The Novartis Foundation The need to better understand missed opportunities at every step of the journey Adapted from Mark McClellan et al. AHA presidential advisory. A call for Action. Circulation 2019 Patient adherence and treatment efficacy Access Provider compliance Absent or low health literacy Failure to make ...
A comprehensive and iterative approach to support Novartis with the development of launch operation plans and tactics. An exciting opportunity with lots of unknowns. Novartis is expanding its portfolio in the cardiovascular space with a first-in-class treatment. As well as being a novel product offering, the intended prescribing customer was ...
Beyond data, information and observations of patients' lives, Novartis started out on a journey to create an effective way of bringing patient insights to the heart of decision-making. This required the development of a creative thinking process in an analytical thinking culture - two fundamentally different types of cognition.
Understanding the real-world patient journey and unmet needs of people with hidradenitis suppurativa through social media research Br J Dermatol . 2023 Jul 17;189(2):228-230. doi: 10.1093/bjd/ljad104.
16 Background: A survey of patients with prostate cancer (PCa) was conducted to map their experiences, expectations and attitudes, and to identify challenges and unmet needs in diagnosis, therapy patterns, care teams, quality of life (QoL), patient organizations and resources. Here, we describe the initial survey results and explore differences in diagnosis and therapy patterns between 3 ...
Novartis reserves the right to rescind, revoke, or amend this program without notice. See complete Terms and Conditions in the enrollment forms for details. If you have already been prescribed PLUVICTO, call for assistance from the Patient Support Team at 1-844-638-7222 ↗ , Monday-Friday, 8:00 AM - 8:00 PM ET, excluding holidays.
The Patient Journey Acceleration (PJA) & New Commercial Partnerships (NCP) Manager acts as the strategic business partner and project manager for the TA with the objective to improve and accelerate the patient journey in the dedicated therapeutic areas and build in-novative partnerships with key accounts and healthcare solutions providers.
Step 1. T cells play a central role in fighting disease, yet they need help recognizing cancer cells. Step 2. At a certified facility, blood from the patient is removed and separated to collect ...
Novartis's patient services also has a set of self-service features that simplify implementing third-party capabilities, offering the best of both worlds. ... customer relationship management applications such as Salesforce to develop custom solutions and deliver the ideal patient journey. For one brand, patients previously took 5.5 minutes ...
Novartis is a world leader in the research, development, manufacturing and marketing of products to protect and improve health and well-being. Our goal is to discover, develop and successfully market innovative products to prevent and cure diseases, to ease suffering and to enhance the quality of life. ... Current patient journey- was developed ...
Introduction Cancer is the leading cause of death worldwide, with breast cancer being one of the most commonly diagnosed types. Low-income and middle-income countries account for nearly half of all breast cancer cases and related fatalities. In Africa, mortality rates are higher and survival rates are lower compared with developed countries. Timeliness of care is a critical aspect of ...
ey: Journey:MOMENTS MOMENTS OF OF CARE CAREA successful patient-centric plan requires two elements: knowing the patie. t and collaboration across all stakeholders.We've all seen them: the patient-cen- tric diagrams that represent as many as seven or even up to 20 stake-holders per patient, all of whom a. e somehow involved in that patient's ...
Follow up in patient´s treatment. •Ideate and lead the 360° competitiveness strategy to accelerate our position and reputation in the Mexican Market / GTx. •Co-create with Key Stakeholders (Private & Public) innovative access models through real world evidence and SMA incidence data generation. Essential requirements:University degree in ...
The data-thirsty personalized medicine market alone was valued at $493.1 billion in 2021 and is expected to grow at a 6.2% compound annual growth rate from 2021 to 2028.1. Elizabeth Theophille, Chief Technology Transformation Officer at Novartis and Dr. Petra Jantzer, Senior Managing Director, Global Accenture Partner for Novartis talk about ...
Developing the future of CAR-T cell therapy today. Novartis is pioneering the way in the class of cell and gene treatment and has developed the first CAR-T cell therapy approved for paediatric and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukaemia (ALL). This heritage includes setting the standard in patient and ...
Location: KanoMajor accountabilities: Drive Competitive Sales Growth -Identify and prioritize high-potential customers through data analysis (HCPs and stakeholders) who influence prescription decisions -Drive sales performance through the skillful orchestration of positive customer experiences -Engage and Build Relationships -Engage in value-based conversations (in-person and virtually) to ...
The impact of PNH. PNH is caused by the premature destruction of red blood cells (RBCs) by a part of the immune system called the complement system. 1-6 Because the main job of RBCs is to carry oxygen - via hemoglobin - to organs and tissues throughout the body, people living with PNH can have low hemoglobin levels, which can lead to debilitating symptoms including anemia. 1,2,6-8 These ...
Negative impact of the IRA on patient access to innovative treatments. Statement Aug 15, 2024. Novartis statement on Maximum Fair Price for Entresto. Story Aug 13, 2024 People and Culture. Every number has a story - my journey to Novartis as a Refugee. Key Release Aug 08, 2024. Novartis receives FDA accelerated approval for Fabhalta ...
• Lead a team of up to 10-12 customer-facing brand specific Case Managers with responsibility of handling all aspects of patient case management including general inquiries, product / program questions, site specific communication preference management and services such as free trialoffer enrollment, co-pay enrollment, adherence enrollment, etc. This work will focus on one of Novartis's ...
Location: The Associate Director, Access & Reimbursement, Radioligand Therapy (RLT) is a remote/field-based role that covers the following cities but not limited to, Houston, Austin, San Antonio, & Corpus Christi TN. The Associate must reside within territory, or within a reasonable daily commuting distance of 60 miles from territory border. Ability to travel and cover geography, at least 50% ...